Automated production technologies for mRNA-based drugs

Needs and challenges
mRNA-based vaccines, along with gene and cell therapeutics, represent innovative drugs that can be used to prevent and/or treat infectious diseases, genetic disorders and cancer. Development of these innovative drugs has been prodigious in recent years in terms of clinical research, translation and application. But advancement of the required production technologies has not yet managed to keep pace with the rapid biomedical progress made in these areas. Hence the need for automated and digitally supported production technologies that facilitate not only the rapid, safe and reliable development of innovative mRNA drugs, but also their production in accordance with the high demands of good manufacturing practice (GMP). The primary objective of the RNAuto lighthouse project is to develop automated manufacturing processes for innovative mRNA molecules that will smooth the way for sustainable and cost-efficient health care.
Biological background
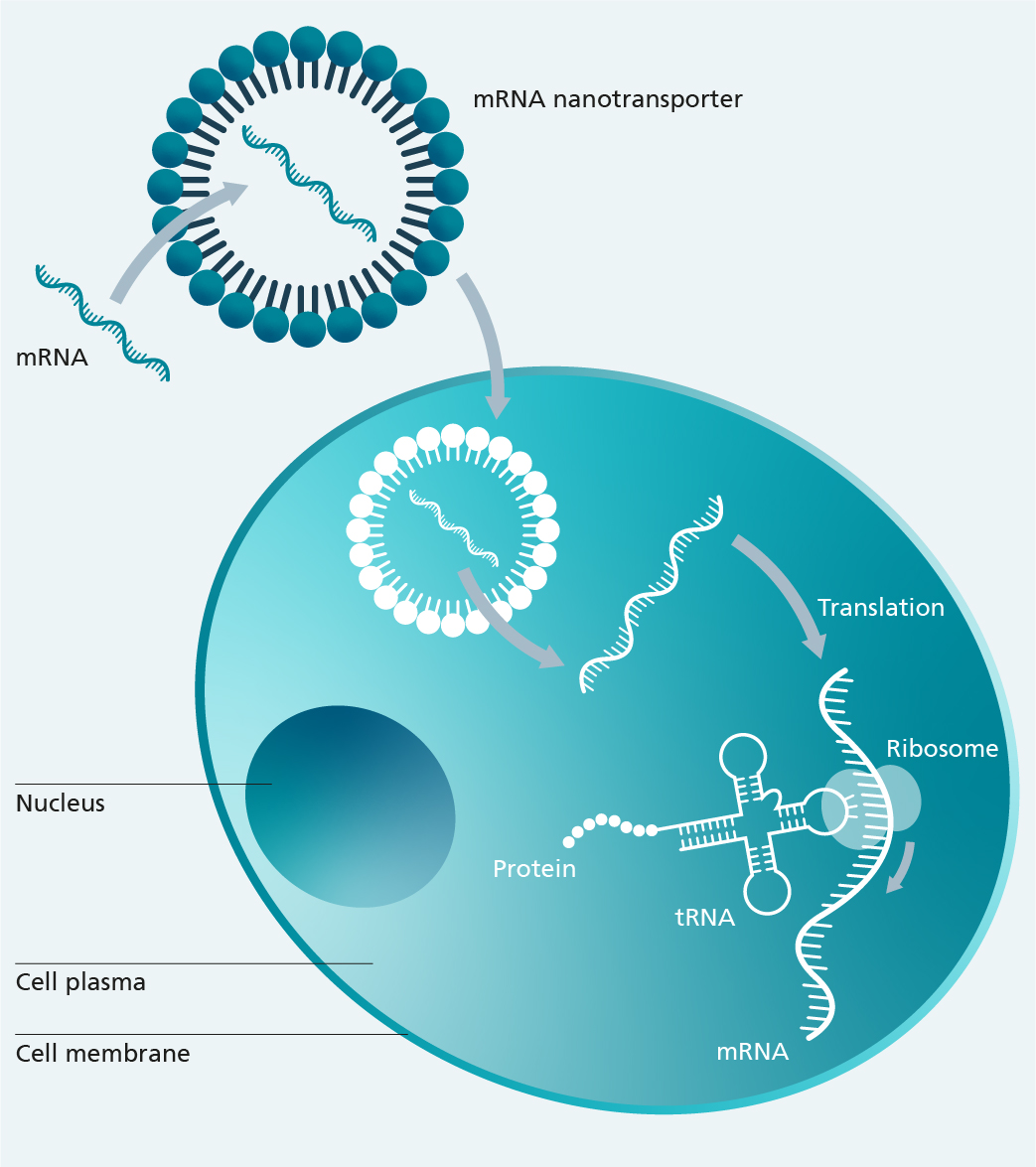
mRNA drugs utilize a mechanism of action that has been in development for three decades, but only received widespread recognition through the SARS-CoV-2 vaccines. mRNA (messenger RNA) molecules are found in all types of cells. It is their job to convey genetic information, the blueprint of a protein, from the DNA located in the cell nucleus to the ribosomes in the cytoplasm, where the information is translated into a functional protein.
In mRNA drugs, the information encodes the protein molecule through the mRNA. The mRNA-based protein produced by the cell then communicates the respective function, e.g. as a prophylactic vaccine or therapeutic protein. This makes the use of mRNA drugs an option in the future wherever proteins play a relevant role in prophylaxis or therapeutic treatment. Besides being used to prevent infectious diseases and as a cancer therapy involving chimeric antigen receptor (CAR) modified immune cells, this may also have a bearing, for example, on monocausal genetic diseases.
The mRNA technology benefits both patient safety and production as it is quicker and more affordable to manufacture mRNA than conventional vaccines. In the field of gene and cell therapy, the fact that mRNAs do not integrate into the genome of patient cells is an advantage. A key challenge facing mRNA technology is the fact that the transfer of mRNA molecules into cells requires transfection systems that allow mRNA to be introduced in a stabilized and efficient way. Biocompatible nanocarriers made of lipids can pack, or encapsulate, mRNA into nanoscopic transport vehicles and facilitate their transfer into cells.
Technical background
In order to encapsulate mRNA in lipid nanocapsules, dissolved lipids are mixed with mRNA to create a mixture which is then buffer-exchanged, concentrated and fractionated. Checking the quality of the encapsulated mRNA by means of dynamic light scattering (DLS) is limited to determining particle size and distribution. In order to manufacture a reliable mRNA drug, consistent product quality has to be ensured that also quantifies the amount of encapsulated mRNA in the nanocarriers. An uneven proportion of encapsulated mRNA at the start of the mixing process and high volumetric flow rates in the production process make it difficult to ensure a continuous quality control. Due to the resulting fluctuations in the viscosity of the mixture, refractive index, conductivity, temperature and pH value, as well as the corresponding impact on product quality, the project addresses not only process optimization, but also the comprehensive quality control of the fractions. Furthermore, the project will establish the continuous production of immune defense cells in a novel bioreactor.
The production technologies currently in use for the scaled, quality-assured and GMP-compliant manufacture of cell therapeutics are inadequate for the planned applications. Semiautomatic, all-in-one devices for manufacturing gene and cell therapeutics present a number of disadvantages in terms of process adjustment and flexibility. There is a lack of in-line solutions for checking the quality of functional cell characteristics. The proportion of manual processes that have to be conducted by qualified staff thus remains very high. Moreover, these devices cannot be used for allogeneic, off-the-shelf cell therapeutics.
One of the project aims is to develop and establish manufacturing processes that can be scaled from clinical to industrial level. To this end, a closed expansion module is being developed that can adjust the culture volume dynamically as the number of cells increases. This module uses a particularly gentle method to cultivate the cells in a sedimentation culture on a double-membrane structure.
In order to achieve optimum integration capacity, functional modules will be created that have a sufficiently large culture surface yet take up just a small space. This will allow allogeneic cell products to be manufactured for 50 to 100 patients in one batch and will enable systems to be easily parallelized, resulting in a more efficient, rapid and affordable procedure.
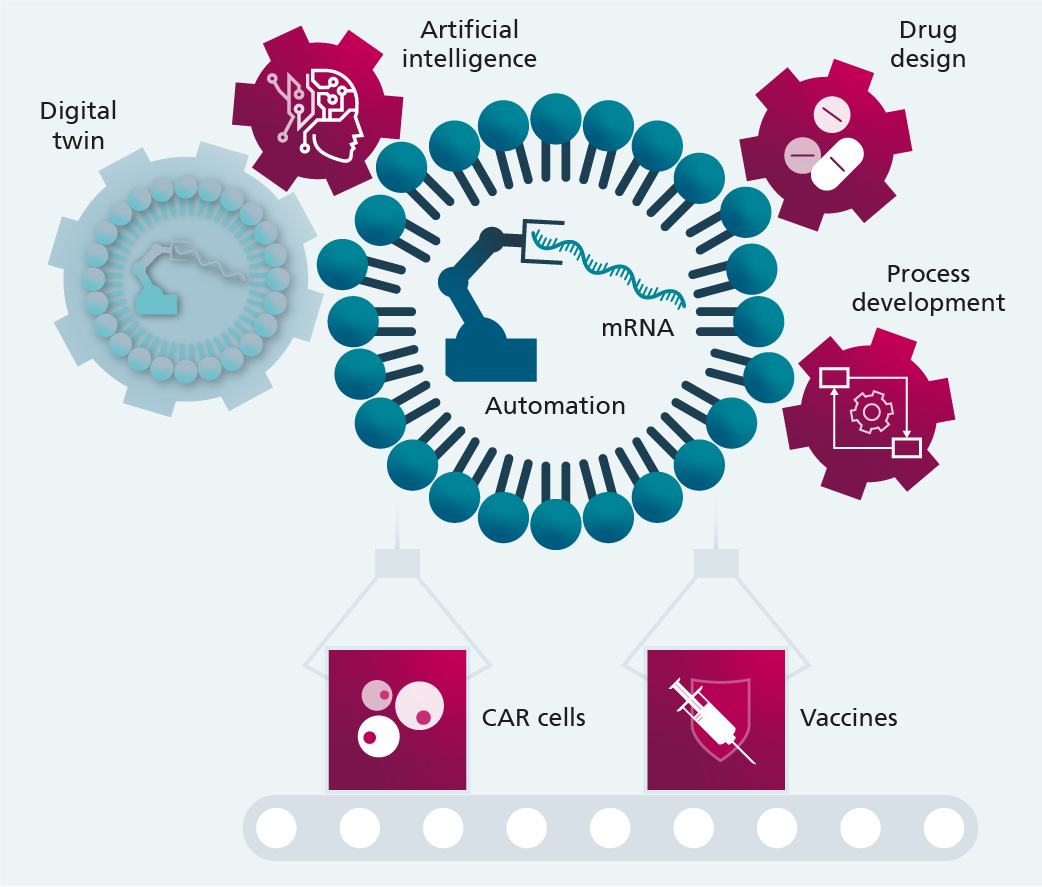
For the automated manufacture of mRNA-based drugs to be successful, technical solutions will be established in the areas of bioreactors, fluid dynamics, quality control and automated data analysis. Key elements of the Industry 4.0 concept are drawn upon to digitally map and monitor the production processes.
The production of advanced therapy medicinal products (ATMP) places huge importance on not only the management of individual devices, but also data integrity. The high complexity of each process step makes recording the required and generated data inconsistent, meaning the data cannot be used later down the line, or it can only be used manually. This limitation can, however, be overcome with the digital twin concept, which enables the relevant data and simulations to be recorded and stored throughout an ATMP’s entire life cycle, allowing all the information needed for production to be utilized. The digital twin can be incorporated in several stages. In the first stage, all product data is recorded in one place over the entire life cycle of an ATMP. This enables consistent documentation and traceability, which help facilitate simplified repeatability – also in other production environments. If data about production and deployed resources are also collected and integrated into the digital twin, the individual production steps can be more easily coordinated. Building on this, process controls are enabled in the next stage that encompass all process steps. This approach can, in turn, help secure process quality and fully automate production; further optimization through the use of artificial intelligence is also conceivable here.
Development goals
This lighthouse project focuses on developing bioprocessing methods and production technologies for the modular and automated manufacture of mRNAs, mRNA nanocarriers and mRNA-modified cells that can be scaled right up to industry level. For the automated manufacture of mRNA-based drugs to be successful, technical solutions will be established in the areas of bioreactors, fluid dynamics, quality control and automated data analysis. Key elements of the Industry 4.0 concept are drawn upon to digitally map and monitor the production processes. The lighthouse project establishes and utilizes practical drug candidates in order to develop the new process and production technologies: the manufacture of mRNA-based vaccines and the manufacture of mRNA-induced gene and cell therapeutics.
Specific project goals
- Automatable process for packing mRNA in nanocarriers
- Development of a screening unit for automated mRNA encapsulation with integrated online analytics for quality assurance and documentation purposes in order to accelerate bioprocess advancement
- Expansion module with integrated quality control for the manufacture of allogeneic gene and cell therapeutics
- mRNA drug candidates: vaccine against West Nile Virus; gene and cell therapeutics against hematological forms of cancer
Participating Fraunhofer institutes
- Fraunhofer Institute for Toxicology and Experimental Medicine ITEM (item.fraunhofer.de)
- Fraunhofer Institute for Microengineering and Microsystems IMM (imm.fraunhofer.de)
- Fraunhofer Institute for Manufacturing Engineering and Automation IPA (ipa.fraunhofer.de)
- Fraunhofer Institute for Production Technology IPT (ipt.fraunhofer.de)
- Fraunhofer Institute for Experimental Software Engineering IESE (iese.fraunhofer.de)
- Fraunhofer Institute for Microelectronic Circuits and Systems IMS (ims.fraunhofer.de)
- Fraunhofer Institute for Cell Therapy and Immunology IZI (izi.fraunhofer.de)
- Fraunhofer Institute for Cell Therapy and Immunology, Branch Bioanalytics and Bioprocesses IZI-BB (izi-bb.fraunhofer.de)