Automated production technologies for mRNA-based drugs
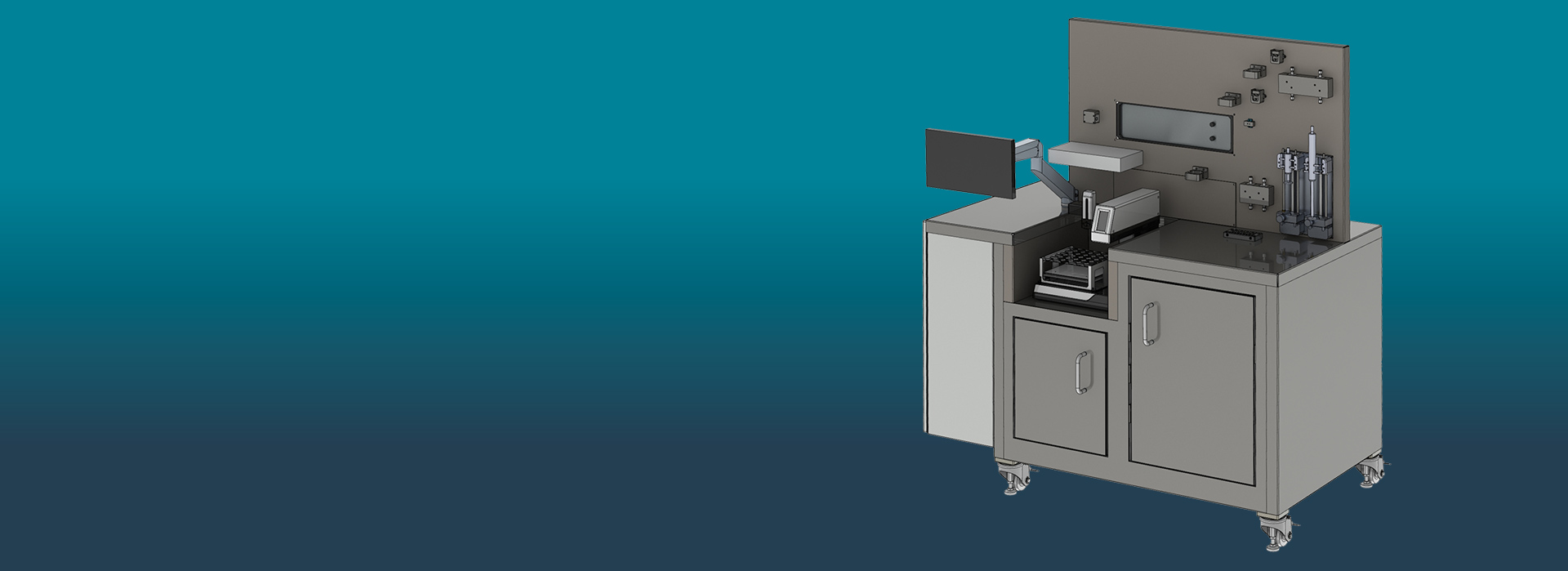
Screening system with digital process control and data-driven inline quality control for automated mRNA nanocarrier production up to 20 ml. The system, measuring 1.40 m x 0.80 m x 1.60 m, consists of scalable, flexibly combinable and manufacturer-independent production modules that can be replaced via plug and play in the event of a defect. Digital quality control is carried out in-line, i.e. without product loss.
Highlights
Production plant
- Modular design made up of individual components
- Scalable and flexibly expandable
- Microfluidic mixing technology with high throughput
- Plug and Play functionality
Process control
- Process control (COPE)
- Digital twins of all individual components (BaSyx)
- Recording of all production parameters
Analysis technology
- Inline dynamic light scattering
- pH value
- Viscosity
- Temperature
- UV/Vis absorption
Inline quality assurance
- Digital twin of product quality (BaSyx)
- Quality control without product loss
- Visualization of all quality parameters in real time
- Digital product passport
- Automated optimization of production parameters
Literature
- Biermann F, Mathews J, Nießing B, König N, Schmitt RH. 2021. Automating Laboratory Processes by Connecting Biotech and Robotic Devices – An Overview of the Current Challenges, Existing Solutions and Ongoing Developments. Processes 9: 966.
- Fischer RF, Graf M, Freundel P, Luetjohann D, Mavreas D, Trapl J, Wolf A. 2021. A Framework for Automated Quality Assurance and Documentation for Pharma 4.0. Lecture Notes in Computer Science (Springer) 12852: 226-239.
- Fischer RP. 2023. A Simplified Integration of Qualified Laboratory Devices with the Asset Administration Shell as the Digital Twin. Concept Paper, Tampa: International Society for Pharmaceutical Engineering (ISPE).
- Fischer RP. 2023. ISPE Baseline Guide: Volume 8 – Pharma 4.0. Guidance Document, Tempa: International Society for Pharmaceutical Engineering (ISPE).
- Hort S, Herbst L, Bäckel N, Erkens F, Niessing B, Frye M, König N, Papantoniou I, Hudecek M et al. 2022. Toward Rapid, Widely Available Autologous CAR-T Cell Therapy - Artificial Intelligence and Automation Enabling the Smart Manufacturing Hospital. Frontiers in Medicine 9: 913287.
- Schmidt A, van Lengen RH, Horbelt J, Gebken N, König N, Biermann F, Blache U et al. 2021. Skalierbare Herstellung von ATMPs - Notwendige Kernbausteine für die standardisierte automatische Produktion von neuartigen Zell- und Gentherapeutika. München: Fraunhofer-Gesellschaft.
- Fraunhofer IMM Nanoparticle Formulation Systems. https://s.fhg.de/IMM-nanoparticle-formulation-systems
- Nanoparticle Sizing: Inline Dynamic Light Scattering. https://s.fhg.de/IMM-nanoparticle-sizing
- IESE BaSyx. https://eclipse.dev/basyx
- IPT Cope. https://cope-software.de