Fraunhofer Institute for Cell Therapy and Immunology in Leipzig celebrates its 20th anniversary
Leipzig, April 29, 2025: The Fraunhofer Institute for Cell Therapy and Immunology (IZI) marked its 20th anniversary in Leipzig, celebrating the milestone with over 400 guests and employees. Invited had been representatives from academia, industry, and politics The event included congratulatory messages from Saxony’s Minister President Michael Kretschmer and Leipzig’s Lord Mayor Burkhard Jung, among others.
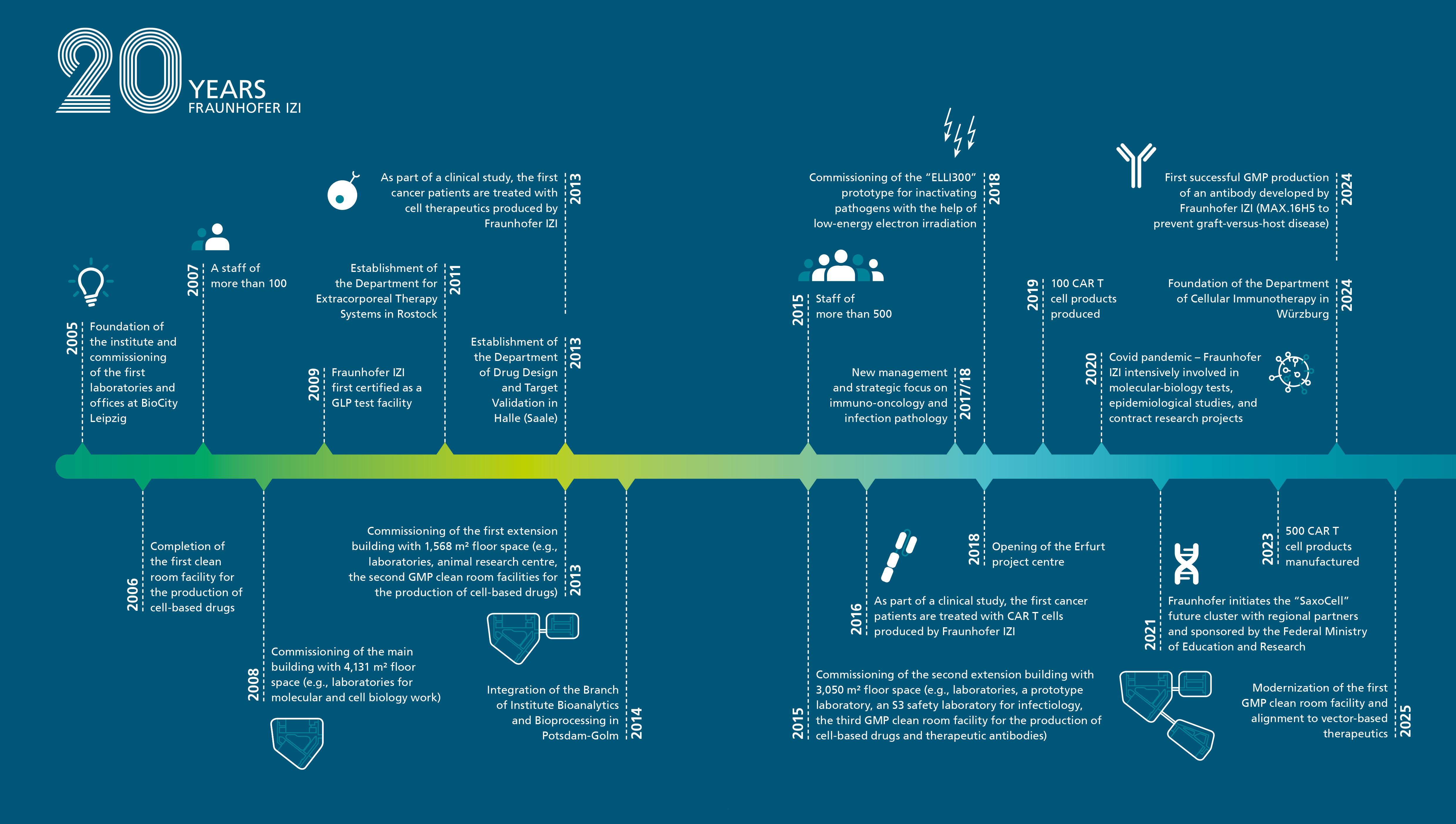
Founded in 2005 with an initial team of 16 employees, the institute began its work in laboratory and office spaces at Leipzig’s BioCity. Its mission centered on bridging the gap between medically relevant basic research and clinical practice through applied biomedical research.
The first decade saw rapid growth, the establishment of a modern research infrastructure, and the development of a diverse portfolio of expertise. Early research focused on regenerative medicine technologies, immune-mediated and degenerative diseases, and vaccine development. Expansion followed with the creation of branch facilities in Rostock (2011) and Halle (Saale) (2013), and the incorporation of the Potsdam-Golm site in 2014, which added expertise in extracorporeal technologies, molecular drug development, and bioanalytics. After a consolidation phase, the institute strategically refocused its efforts in 2018 on immuno-oncology and infection pathology. Today, the Fraunhofer IZI employs approximately 600 people and manages an annual project volume of around €40 million.
The Fraunhofer IZI develops, optimizes and validates processes, materials and products for biotechnological, pharmaceutical and medical technology companies, clinics, diagnostic laboratories and academic research institutions across its core business units Cell and Gene Therapy, Drugs and Vaccines, Molecular and Immunodiagnostics and Extracorporeal Therapies.
The institute gained international recognition through its role in producing clinical investigational medicinal products for the landmark clinical trial that led to the first European approval of CAR-T cell therapy. This achievement marked a milestone in modern oncology. To date, the institute’s GMP-compliant cleanroom facilities have manufactured over 600 CAR-T cell products for clinical trials
Another breakthrough is the development of low-energy electron irradiation (LEEI) technology for pathogen inactivation. This innovation enables more efficient and cost-effective production of inactivated vaccines and holds promise for applications such as irradiating immune cells in cell-based immunotherapies.
Additionally, Fraunhofer IZI has contributed to over 100 R&D projects, consistently driving advancements in next-generation medical solutions.