Automated production technologies for mRNA-based drugs
In a world where innovation and efficiency go hand in hand, Pharma 4.0 is revolutionizing the way we make medicines. Our Pharma 4.0 packages are specifically designed to optimize your production processes, enhance product quality and shorten time to market. Choose the right offer for your individual needs.
Package 1: Pharma 4.0 potential analysis
Find out how you can minimize manual processes and maximize the efficiency of your production lines by using state-of-the-art technologies and intelligent automation. Our potential analysis includes:
- Technological and economic analysis of your manufacturing process
- Gap and potential analysis
- Clarification of the regulatory framework
- Development of an implementation strategy tailored to your company

Package 2: Individual components for production
Our scalable and flexible solutions can be easily integrated into existing systems, enabling you to quickly reap the benefits of digitalization. Our services include:
- The design and development of individual components (formulation modules, downstream processing and process analytical technology)
- The creation of interface solutions with digital twins
- Seamless integration into existing production plants

Package 3: Component-based production systems
Realize your vision of a digital production system with our scalable and flexible solutions. We offer you:
- Digital modeling of your production process
- Design and creation of an automation concept
- Integration of inline QA measures into your production process
- Automated documentation of the achieved product quality
- Complete system planning and commissioning
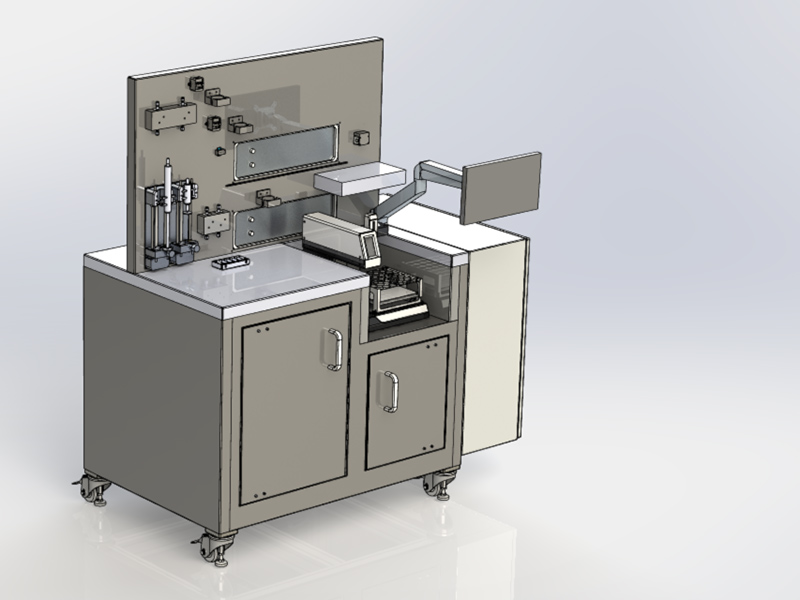
Package 4: Digital Quality Management
Optimize your quality management in pharmaceutical manufacturing with our digital solutions that meet the highest safety and compliance standards. We support you in:
- Formalizing a continuous digital quality management process
- Integrating it into the existing production process
- Using our software solutions to specify digital quality risk models

Take the next step into the future of pharmaceutical production! Contact us today to learn more about our digital offerings and how we can help you achieve your production goals. Together, we can shape the pharmaceutical industry of tomorrow!
Literature
- Biermann F, Mathews J, Nießing B, König N, Schmitt RH. 2021. Automating Laboratory Processes by Connecting Biotech and Robotic Devices – An Overview of the Current Challenges, Existing Solutions and Ongoing Developments. Processes 9: 966.
- Fischer RF, Graf M, Freundel P, Luetjohann D, Mavreas D, Trapl J, Wolf A. 2021. A Framework for Automated Quality Assurance and Documentation for Pharma 4.0. Lecture Notes in Computer Science (Springer) 12852: 226-239.
- Fischer RP. 2023. A Simplified Integration of Qualified Laboratory Devices with the Asset Administration Shell as the Digital Twin. Concept Paper, Tampa: International Society for Pharmaceutical Engineering (ISPE).
- Fischer RP. 2023. ISPE Baseline Guide: Volume 8 – Pharma 4.0. Guidance Document, Tempa: International Society for Pharmaceutical Engineering (ISPE).
- Hort S, Herbst L, Bäckel N, Erkens F, Niessing B, Frye M, König N, Papantoniou I, Hudecek M et al. 2022. Toward Rapid, Widely Available Autologous CAR-T Cell Therapy - Artificial Intelligence and Automation Enabling the Smart Manufacturing Hospital. Frontiers in Medicine 9: 913287.
- Schmidt A, van Lengen RH, Horbelt J, Gebken N, König N, Biermann F, Blache U et al. 2021. Skalierbare Herstellung von ATMPs - Notwendige Kernbausteine für die standardisierte automatische Produktion von neuartigen Zell- und Gentherapeutika. München: Fraunhofer-Gesellschaft.
- Fraunhofer IMM Nanoparticle Formulation Systems. https://s.fhg.de/IMM-nanoparticle-formulation-systems
- Nanoparticle Sizing: Inline Dynamic Light Scattering. https://s.fhg.de/IMM-nanoparticle-sizing
- IESE BaSyx. https://eclipse.dev/basyx
- IPT Cope. https://cope-software.de