Department of Extracorporeal Therapy Systems
ADsorb – Development of an implantable system for liquorpheresis in neurological diseases using the example of Alzheimer’s dementia
At the present time, Alzheimer’s dementia (AD) is one of the biggest medical challenges in our society. So far, this disease can neither be cured nor can its progression be halted effectively.
The accumulation of intracerebral beta-amyloid within the central nervous system (CNS) and the cerebrospinal fluid correlates with the characteristic symptoms, such as memory loss and, in part, pronounced behavioral changes. Therefore, the reduction of this neurotoxic protein through liquorpheresis appears a promising therapeutic approach. This approach is based on extracorporeal blood purification therapies which are used, in particular, to treat acute and chronic renal and liver failure as well as autoimmune disorders. These methods are based on the targeted reduction of pathogenic molecules which would otherwise accumulate in tissues and contribute to the progression of the illness. In this context, liquorpheresis could help to balance the age- or illness-related dysfunction of the liquor system and, as a result, to slow down and, in the best case, stop the progression of the illness.
The project aims to develop and research a liquorpheresis technology for the removal of specific target proteins which are generated in the CNS from the cerebrospinal fluid. To this end, a model system will initially be developed in the transgenic Alzheimer’s mouse to try out a miniaturized, implantable liquorpheresis device. Fraunhofer IZI will contribute its expertise in the field of extracorporeal technologies and adsorbers.
Partners
Rostock University Medical Center; University of Rostock
Support
Go-Bio initial program / Federal Ministry of Education and Research
![BMBF_CMYK_Gef_M [Konvertiert]](/en/departments/rostock-location/extracorporeal-therapy-systems/projects/jcr:content/contentPar/sectioncomponent_1692061379/sectionParsys/imagerow/imageComponent1/image.img.jpg/1748249868293/BMBF-gefoerdert-2017-en.jpg)
Pathological blood-brain barrier model for the development of novel treatment strategies
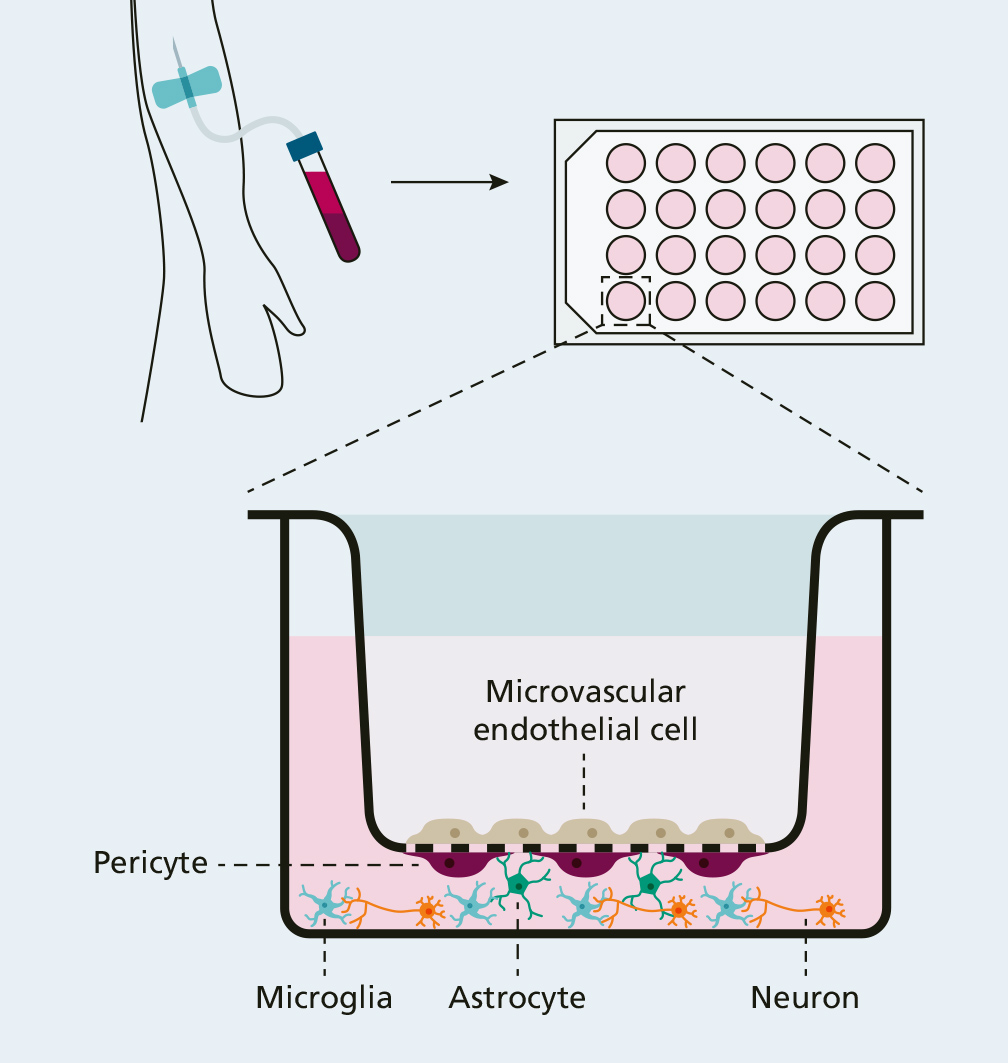
The blood-brain barrier is one of the most impermeable protective barriers of the body. It protects the sensitive central nervous system against both cytotoxic influences and blood-borne pathogens. The barrier consists of microvascular endothelial cells, pericytes and astrocytes which form a neurovascular unit together with neurons and microglia.
In the event of a systemic disease, such as sepsis (blood poisoning), however, this barrier is weakened by the accumulation of harmful substances in the blood, which, in turn, can result in long-term neurological damage. For example, more than one half of patients affected by sepsis suffer from secondary cognitive and psychological conditions - even one year after the onset of the disease. Because of the high complexity of the pathophysiological processes in the body, specific neuroprotective treatment options for patients suffering from acute sepsis or organ failure have not yet been developed.
In cooperation with the University Medical Centre Rostock, Fraunhofer IZI is developing a human in vitro disease model of the blood-brain barrier based on induced pluripotent stem cells (iPS). As part of this, the cell types of the neurovascular unit – endothelial cells, pericytes, astrocytes, neurons and microglia – are created from stem cells by means of specific differentiations. These are brought together in physiological arrangement in a transwell system modelling the “healthy” blood-brain barrier. Pathological conditions under which the blood-brain barrier can possibly be damaged can then be simulated by incubating plasms from sepsis or organ-failure patients.
Afterwards, the damage can be examined and characterized both at a cellular and molecular biological level. This project aims to establish a meaningful cell model for the development of protective agents and drugs.
The project is supported by the Damp-Stiftung.
Partner
University Medical Centre Rostock; NeuroProof Systems GmbH
Ex situ organ perfusion
The availability of suitable donor organs for patients with organ failure has been a major problem for many years. On the one hand, the number of donations itself is too low – it is estimated that only about ten percent of the actual demand can be met with transplanted organs. On the other hand, the functionality of donated organs or the health status of potential donors often does not meet medical requirements. Pre-existing diseases of the donor, the presence of risk parameters, especially in the case of livers, e.g. a high degree of adiposity, but also the duration of the ischemic period (lack of blood and oxygen supply to the organ during transport) after organ removal can lead to considerable organ functional losses. Such "marginal" organs usually mean a lower probability of survival for the transplanted patient, or are not even considered as donor organs. However, due to the high demand for donated organs, there is a growing desire to also consider marginal organs for transplantation.
This is where the "OrganFit" project comes in. It is already known from published work that organs can be kept functional for a certain period of time by means of machine perfusion. If the perfusion is then carried out at physiological temperatures ("normothermic"), the preservation of function is improved compared to hypothermic conditions, as the cold ischemia time is reduced.
The organ perfusion platform established in the Department of Extracorporeal Therapy Systems in Rostock will now be used to investigate further approaches that will support the use of marginal livers. For this purpose, both technical and biological approaches are planned to support or even strengthen the natural regeneration potential of livers. Livers from pigs will initially be used to establish and test these approaches. The project is carried out in close cooperation with the Clinic for Transplantation Surgery and the Clinic for Anaesthesia and Intensive Care Medicine of the University Medicine Rostock.
Looking beyond the current project, the technical platform should also enable new cooperations and further research projects and thus make the most diverse use of the opportunities offered by ex situ organ perfusion.
Cryoregeneration of dialysate
Patients whose bodies have a weakened detoxification function due to a late-stage chronic kidney disease regularly need to have dialysis. The principle behind this procedure has been established for decades and is based on extracting water-soluble toxins (uremia toxins) in an extracorporeal filter, i.e. the dialyzer. The toxins pass from the blood into the purifying dialysis water (dialysate) via a membrane in the dialyzer. Around 120 liters of dialysate are required for every dialysis treatment, which usually takes four hours and is repeated three times a week. This water is taken from reverse osmosis (RO) plants in hospitals and specialized dialysis practices. Not only do these plants take up a lot of space and energy, but the water can only be used once as it disappears as waste water following dialysis. Based on a one-year time frame and 90 000 patients in Germany, over 1.7 million cubic meters of highly purified water are needed, without even taking the lost RO water into account.
Based on an approach which has never been applied to dialysis in the past, a procedure is being developed in the Department of Extracorporeal Therapy Systems that facilitates the regeneration of used dialysis water and could therefore completely change the huge problem of water dependability affecting the use of dialysis today. This procedure draws on the concept of freeze concentration used in the beverage industry and is based on the principle that the crystal lattice structure of frozen water excludes any previously dissolved foreign substances. As part of an automatable cycle, the procedure enables contaminated dialysate to be separated into pure water and a small residual volume containing impurities. This residual volume may arise from the patient’s regular liquid intake, removing the dialysis’ dependency on a pure water supply and making entirely mobile solutions conceivable. The fact that this separation occurs regardless of substance properties such as solubility, polarity, size, density, etc. is hugely beneficial compared with all conventional filter-based procedures, which do not demonstrate a sufficient filter capacity, especially for urea.
The procedure is currently being patented and an automated solution is undergoing development. This technical solution will then be used to carry out extensive investigations to specify process parameters that can later be used when working towards the initial clinical application. Notable industrial companies have already shown an interest in the procedure despite it’s early stage of development.
3D Tissue Model Systems
This sub-project forms part of the HOGEMA joint project: "Researching novel approaches to provide improved tissue replacement materials based on hydrostatic high-pressure treatment".
The project sees partners from the University of Rostock, Greifswald University Hospital, Wismar University of Applied Sciences, Fraunhofer IZI and the University of Rostock's Department of Medicine working together to explore new ways of providing more effective tissue replacement materials based on hydrostatic high-pressure treatment.
Fraunhofer IZI’s Department of Extracorporeal Therapy Systems will investigate the applicability of hydrostatic high-pressure technology in rodents, looking at soft tissue such as the kidneys or intestines. The aim here is to produce tissue scaffolds with a much higher matrix integrity than when using wet chemical methods. By subsequently repopulating the scaffolds with human cells, these models can be used for a number of medical, pharmacological and biotechnical investigations.
More information on the "HOGEMA" research alliance can be found at: hogema.med.uni-rostock.de
The project is supported using funds provided by the European Social Fund (ESF) within the scope of the qualification program "Promotion of junior scientists in outstanding research alliances – excellence in research program of the state of Mecklenburg-Western Pomerania".
Funding reference number: ESF/14-BM-A55-0012/18


Renal tissue models
Today, approx. ten percent of the global population are affected by chronic kidney disease (CKD). The current treatment options comprise dialysis and kidney transplants which, however, are unsatisfactory both medically and in terms of the patients’ welfare for several reasons. Therefore, the possibility of a future third option by providing synthetic (bioartificial) kidneys would definitely be very valuable. While impressive progress has been made with research approaches from the field of tissue engineering, the road to functional kidneys seems to be very long. This project aims to establish, primarily, tissue model systems which can be used to address different questions more specifically regarding the efficient decellularization and subsequent recellularization of kidney tissue from rats. After the removal of the animal cells, an extra-cellular matrix (ECM) remains in the form of a delicate, anatomically intact scaffold forming the basis for colonization by human cells. However, both processes – i.e. the removal of the original cells as well as the recolonization with human cells – are complex procedures which can be significantly optimized in certain details. Up to now, perfusion of the organ with chemical reagents has been predominantly used for decellularization. However, this can also lead to a significant impairment of the quality and integrity of the remaining scaffold. For the first time in this project, the application of hydrostatic high pressure treatment (HHD) to the decellularization process is investigated. HHP can lead to a very fast and effective devitalization of the cells so that subsequent perfusion would be shorter and less harmful for the ECM which, in turn, would be very advantageous for recellularization. In addition to intact kidneys, precision-cut kidney sections, which are excellently suited as 3D tissue model systems, are examined in this context.
Finally, this type of tissue model is to be used for a range of questions – from the precise examination of cellular and molecular processes during recellularization, to functional analyses of nephrons and pharmacological issues. The project is sponsored in the framework of the Mecklenburg-Vorpommern state initiative for excellence (HOGEMA consortium). The tissue replacement material research partnership brings together the universities of Rostock and Greifswald as well as Wismar University of Applied Sciences.


MedTech – Further projects in the field of medical technology
The MedTech area focuses on the development and testing of innovative therapies, in particular those which aim to support the immune system. Novel treatment strategies are developed on the basis of classic extracorporeal technology platforms, such as dialysis and plasma separation. Special emphasis is placed on the development of extracorporeal blood treatment methods for sepsis therapy. Another approach is the development of extension systems for dialysis.
Development of the extracorporeal immune support system (EISS)
Over 18 million people around the world suffer from severe sepsis every year. In Germany alone, up to 60,000 people die from the disease every year. This is why, together with the company Artcline GmbH, the Department of Extracorporeal Therapy Systems has developed and evaluated a cell bioreactor system which can be used to treat sepsis using immune cells (granulocytes) taken from healthy donors. In doing so, the patient's plasma is circulated extracorporeally in a bioreactor with a granulocyte concentrate taken from a donor of a compatible blood group. During this process, bacterial toxins and other inflammatory substances are removed from the blood plasma by means of phagocytosis, allowing the purified patient plasma to be fed back into the patient's bloodstream. Initial clinical results show a clear improvement in patients' cellular immunocompetence, alongside the decrease in bacterial components. Further clinical trials with adjusted parameters are planned for the near future.
Granulocyte purification
This project looks at optimizing the purification of granulocytes, which are required for the extracorporeal immune support system. A key goal here is to extend storage time under currently applicable transfusion medicine conditions. At present, granulocyte concentrates can only be stored for up to 24 hours at 20–24 °C. An increase in the durability of granulocyte concentrates would significantly increase the flexibility of the therapies to be conducted in an everyday clinical setting and also the logistics associated with donations. Furthermore, pure granulocyte concentrations cannot be manufactured at present as the preparation becomes contaminated by a certain amount of erythrocytes and thrombocytes during apheresis. This project therefore also aims to process human blood preparations in such a way that allows the purest granulocyte fraction possible to be obtained and, in turn, optimally used in a clinical and therapeutic context.
Cytokine adsorber
Sepsis, also commonly referred to as blood poisoning, is a complex systemic inflammatory response produced by the body. It is usually triggered by bacteria and their toxins when they manage to enter the bloodstream and results in a powerful immune response which is also directed in part against the patient’s own body. In severe cases, the patient becomes highly susceptible to secondary infections and multiple organ failure. Sepsis and its serious forms of progression is becoming more and more prevalent around the world. This explains why severe cases of sepsis still prove fatal in over 50 per cent of cases today, in spite of the various state-of-the-art intensive care options available. It is essential that the disease is diagnosed and effective antibiotic treatment initiated as quickly as possible: this can mean the difference between life and death. In order to improve a patient’s prognosis, various therapeutic approaches such as renal replacement therapy or adsorptive procedures can be introduced in addition to standard treatment. Hemoperfusion is an important adsorptive procedure, whereby the patient’s blood passes extracorporeally through a cartridge containing an adsorbent. The various commercially available adsorbers target either the removal of toxins (lipopolysaccharides) or of inflammatory mediators (cytokines / chemokines), which are increasingly released during the overreaction of the immune system. This type of cytokine adsorber (CytoSorb) was examined in collaboration with the company CytoSorbents Europe GmbH. CytoSorb adsorption columns are approved in Europe as an auxiliary extracorporeal treatment for sepsis. The columns aim to remove pro- and anti-inflammatory messenger substances from the blood during the immediate defense response where possible. As, however, this adsorption is a non-specific process, investigations were carried out into whether and to what extent different medications used in intensive care such as antibiotics and also other substances such as painkillers, sedatives, cardiovascular drugs and anticoagulants are similarly retained by the adsorber. In in-vitro studies it could be shown that the investigated adsorber removes a significant amount of an anticoagulant-inhibitor (rivaroxaban) from the blood. This finding is of direct clinical relevance: patients who take this anticoagulant-inhibitor and require emergency surgery cannot be subjected to surgery before the active agent has been completely excreted from the body – a process that can take up to eight hours. The adsorber could notably reduce this waiting time, thereby gaining valuable therapy time. Further investigations into the removal of various other interesting substance classes are scheduled for 2018.
CellTech – Further projects in the field of cell and biotechnology
The CellTech area primarily evaluates the therapeutic and toxic effects (hepatotoxicity) of innovative active ingredient components with the aid of in vitro and in vivo models.
Biosensor technology for the prevention or early detection of liver failure
Liver failure is associated with a high mortality rate and can be caused by acute liver diseases or by the deterioration of an existing liver disease. However, medication such as paracetamol may also cause acute liver failure. Liver damage caused by medication is the most common reason for withdrawing drugs that have already been approved for the market. However, there is no reliable test system available at present to detect liver failure at an early stage. This gave rise to the development of a microtiter plate assay based on human liver cells that can be used to detect liver failure at an early stage in a clinical setting and to evaluate the toxicity of drugs and medical devices. By optimizing and standardizing the procedure, reliable statements can be made with regard to exogenous, and also endogenous, toxicity.
Clay minerals in the treatment of CKD and CIBD
The use of clay minerals represents an innovative therapeutic approach in the treatment of chronic inflammatory bowel disease (CIBD). The therapeutic and prophylactic potential of the envisaged clay minerals is currently being characterized in detail with the aid of cell-based models and animal trials. Besides the positive therapeutic and prophylactic effects in the case of CIBD, clay minerals have already been proven to have no toxic potential. The applied clay minerals are assumed to have effects which strengthen the intestinal barrier, such as an antioxidant potential, as well as the ability to bind endotoxins and thus support the treatment of CIBD.
An additional field of application uses clay minerals as phosphate binders in patients suffering from chronic kidney disease (CKD). Patients with renal insufficiency often suffer from much higher phosphate concentrations in the blood serum, which can lead to cardiovascular diseases. To counter this, the phosphate-binding capacity of clay minerals is currently being investigated in a new therapeutic approach with regard to the prevention of cardiovascular diseases. The therapeutic potential is currently being analyzed using an in vitro calcification model for human coronary artery smooth muscle cells (HCASMC) and also in animal trials involving rats with chronic renal failure (5/6 nephrectomy, 5/6 NX).
ECOS – Study center for extracorporeal methods and biosimulation
In order to translate the scientific concepts of the project group into clinical applications in a timely manner, the ECOS study center has now been integrated into the group. On the one hand, the study center will be dedicated to implementing its own scientific concepts and developments in practice; on the other, it shall conduct clinical and preclinical trials in the field of medical extracorporeal therapies and systems on behalf of industry partners and in close cooperation with the University of Rostock's Department of Medicine (UMR) and local hospitals. The research concerns both interventional and non-interventional trials with drugs and medical devices. Our chain of competence here covers all aspects of clinical trials, from planning over to conduct and monitoring through to biometric evaluation and publication. Another key research area lies in analyzing the spread, causes and processes as well as the influencing factors and interactions of diseases in the field of indication, based on clinical and representative population data.
At present, ECOS is involved in a multicenter clinical trial investigating the efficacy and safety of the extracorporeal liver assist device ELAD® in patients with severe alcoholic hepatitis. Two additional observational studies will investigate the effectiveness of using different adsorbers in albumin dialysis and / or for the treatment of patients with severe sepsis.