Contact Press / Media
Dr. Sina Riemschneider
Head of Toxicology and Immunotoxicology Unit
Fraunhofer Institute for Cell Therapy and Immunology
Perlickstraße 1
04103 Leipzig, Germany
Phone +49 341 35536-1260
Glycosaminoglycans (GAGs) are a critical component of the extracellular matrix in animal tissues and are of essential importance for the structure and function of connective tissues, such as bones and cartilage, as well as of synovial fluid. Various studies have shown that GAGs play a key role in signal transduction for regulating the activity and interaction of cells. As a result of these studies, the Wnt signal path has been identified as being an effective target to increase bone formation. To facilitate this, novel modified GAGs have been developed in a cooperative project. These were then synthesized at the Free University of Berlin and first tested successfully at Dresden Technical University (REGAGs = rational engineered glycosaminoglycans).
As part of this project, Fraunhofer IZI and the Bone Lab of Carl Gustav Carus University Hospital in Dresden are evaluating two drug candidates for their osteogenic effectiveness and safety - in accordance with harmonized standard operating procedures (SOP) to prepare for clinical development. The tests are conducted using a murine calvarial defect model in which REGAG functionalized scaffolds are used to regenerate bone.
In parallel with preclinical studies, the REGAG synthesis (Free University of Berlin) is being developed further, while the detailed in-silico and in-vitro characterization of molecular interactions of the agents with the desired target proteins, as well as potential off-targets, is carried out (Dresden Technical University). At Fraunhofer IZI, proteomic analyses of regenerated bones and of the blood are being used, in addition, to examine the effectiveness and tolerability of REGAGs at the molecular level.
Project partners
Dresden Technical University (coordination), Biotechnological Centre and Max Bergmann Center of Biomaterials; Free University of Berlin; Free University of Berlin, Institute of Pharmacy
![BMBF_CMYK_Gef_M [Konvertiert]](/en/departments/leipzig-location/preclinical-development-and-validation/projects/jcr:content/contentPar/sectioncomponent_511/sectionParsys/imagerow_copy_copy_c_1375318556/imageComponent1/image.img.jpg/1718625172392/BMBF-gefoerdert-2017-en.jpg)
Chronic inflammatory bowel diseases (IBD) are recurrent or persistent inflammatory intestinal diseases. In Germany, around 320,000 patient suffer from the two most common forms of these diseases, ulcerative colitis and Crohn’s disease. The inflammatory reactions cause lasting damage to the intestinal mucous membrane, which, in many cases, results in serious health problems, such as abdominal pain, diarrhoea and blood in the stool.
In spite of intense research, the specific causes and triggers of chronic inflammatory bowel diseases have not yet been fully established. The current state of research assumes that these diseases are likely to be caused by a complex interaction of genetic and environmental factors, plus the composition of the microbiome, as well as abnormal immune responses. So far, the therapeutic options comprise almost exclusively anti-inflammatory medication (e.g. amino salicylates, glucocorticoids, and immune modulators), the long-term use of which involves considerable side-effects.
Fraunhofer IZI is studying and developing novel agents, whose mechanism is based on the binding and activation of the aryl hydrocarbon receptor (AhR). AhR is, e.g., expressed by epithelial cells in barrier tissues (especially in the intestine, lungs and skin), as well as by different types of immune cells, and plays an essential role in regulating the immune response. Therefore, the receptor or corresponding ligands are promising target structures for the development of new drugs.
In explorative studies, drug candidates have been identified from among numerous AhR-binding molecules. These candidates have shown a promising effectiveness and toxicity profile in subsequent proof-of-concept studies. As part of this project, the safety and effectiveness of one of these drug candidates is to be proven and the preconditions for an early clinical study (phase 1/2a) in humans are to be created in a multi-centre confirmatory preclinical study.
This confirmatory preclinical study will be implemented with two study arms – one at Fraunhofer IZI and one at the Department of Medicine of the Otto von Guericke University Magdeburg. To this end, an animal model of mice developed by Magdeburg University will be used to simulate the pathological situation in chronic colitis and evaluate therapeutic interventions. In turn, Fraunhofer IZI will contribute its comprehensive expertise in implementing preclinical studies in accordance with the regulatory quality requirements of good laboratory practice (GLP) as well as its long-standing experience in researching AhR.
In addition to the investigation and assessment of clinically relevant parameters, further analyses, e.g. differential spatial transcriptome analyses of the intestinal tissue, are to be undertaken to establish the mechanism behind the newly developed AhR ligands.
Project partners
Otto von Guericke University Magdeburg, Department of Medicine, Institute of Medical Microbiology and Hospital Hygiene; Fraunhofer Institute for Integrated Circuits IIS
![BMBF_CMYK_Gef_M [Konvertiert]](/en/departments/leipzig-location/preclinical-development-and-validation/projects/jcr:content/contentPar/sectioncomponent_578011992/sectionParsys/imagerow_copy_copy_c/imageComponent1/image.img.jpg/1718625172392/BMBF-gefoerdert-2017-en.jpg)
CAR-T cell therapy is based on the principle of equipping immune cells (T cells) with a chimeric antigen receptor (CAR) by genetic modification. This enables the immune cells to identify specific surface structures (antigens) on cancer or other target cells and to activate a corresponding immune response. In the therapies approved to date, the T cells are modified using viral vectors. With the ROR2-CAR-T cell therapy, scientists at the University Hospital of Würzburg have developed an immunotherapy that differs from previously approved therapies both in the type of genetic modification and the target antigen addressed. This is now to be transferred to clinical application as part of a project funded by the German Federal Ministry of Education and Research. The ROR2 protein is a transmembrane receptor that plays an important role especially during embryonic development. It is normally not expressed, or only very slightly expressed, in non-malignant cells and tissues. However, in some cancers, including multiple myeloma and clear cell renal cell carcinoma, it is overexpressed on the cancer cells in question. This makes the antigen a suitable target for appropriately targeted CAR-T cells. In this project, a new method for the production of autologous CAR-T cells is used. The genetic modification of the patient's own T cells is carried out via a non-viral gene transfer, which, compared to viral gene transfer, will enable a simpler, more scalable and thus less expensive production process. The chimeric antigen receptor was designed to initiate overexpression of the transcription factor Batf3 in addition to T cell activation to improve T cell persistence and tumoricidal activity.
Fraunhofer IZI is responsible for two main aspects of the project: (1) preclinical safety and effectiveness testing of the novel CAR-T cell product, including the determination of the expression of the ROR2 target molecule in healthy tissues and the identification of potentially cross-reacting epitopes (Tissue Cross-Reactivity GLP Study) and (2) the pharmaceutical production of clinical investigation drugs for the clinical study, including the prior establishment and validation of the production process, as well as of the safety-relevant quality controls. The multi-centre clinical study (phase I, first-in human) is carried out at the university hospitals in Würzburg, Regensburg and Leipzig.
![BMBF_CMYK_Gef_M [Konvertiert]](/en/departments/leipzig-location/preclinical-development-and-validation/projects/jcr:content/contentPar/sectioncomponent_2091472598/sectionParsys/imagerow_copy/imageComponent1/image.img.jpg/1715948650034/BMBF-gefoerdert-2017-en.jpg)
This project focuses on developing and producing new kinds of fully humanized monoclonal antibodies for the treatment of tumour diseases (pilot project: triple-negative breast cancer). The development and production of such antibodies can be divided into several steps: The first step entails establishing a humanized mouse model which can be used to generate human monoclonal antibodies against known and yet unknown tumour antigens. A variety of immunization strategies have been created for this purpose in immune-deficient NSG or BRGS mice, which have developed a humanized immune system after the transfusion of human haematopoietic stem cells from cord blood. Tumour-specific human monoclonal antibodies are generated by fusing tumour-specific human B cells from these humanized mice with human plasmocytoma cells, and selected using suitable strategies. Selected candidates are finally tested in an established tumour mouse model in preclinical studies and modified if required (e.g. antibody-drug conjugates), in order to continue improving therapeutic efficacy.
A cooperation project with Regensburg University has already shown that human tumour-specific IgM and IgG antibodies form in humanised mice and can be verified in the serum after the co-transplantation of human haematopoietic stem cells from the umbilical cord and human breast cancer cell lines.
Supported by: EFP
As part of a joint project funded by the BMBF, a combined drug product for pain relief is being investigated in preclinical studies. The aim is to minimize safety risks prior to first-time use in patients. The NANOpain project is based on a combination of an already approved opioid (preferably kappa-receptor agonist) and a dendritic nanotransport molecule (nanocarrier). According to the EPR effect (”enhanced permeability and retention effect”), the dendritic molecules preferentially accumulate in inflamed and tumorous tissue resulting in a targeted localization and a reduction of side effects such as addiction, aversion and constipation. The efficacy has already been tested by DendroPharm both in vitro and in vivo. At Fraunhofer IZI, the safety-relevant preclinical investigations are carried out in appropriate animal models (small animal model, large animal model) under GLP conditions. First, a pharmacodynamics / pharmacokinetics study is performed in rats to investigate the degradation of the drug as a function of time. The toxicological tests of the active ingredient preparation are then implemented in a mouse model as well as in a minipig model. Together with the production of the investigational drug under GMP conditions by DendroPharm, this creates the prerequisite for subsequently testing the developed drug in a Phase I clinical trial at the Fraunhofer Institute for Toxicology and Experimental Medicine.
![BMBF_CMYK_Gef_M [Konvertiert]](/en/departments/leipzig-location/preclinical-development-and-validation/projects/jcr:content/contentPar/sectioncomponent_465128515/sectionParsys/imagerow/imageComponent1/image.img.jpg/1715948944915/BMBF-gefoerdert-2017-en.jpg)
The designation “chronic inflammatory bowel disease” (IBD) comprises pathologies characterised by intermittent or continuous inflammatory changes to the intestinal epithelium. The most important IBDs are Crohn’s disease and ulcerative colitis. In Germany, there are around 300,000 people who suffer symptoms like stomach pains, diarrhoea, exhaustion and eye and joint inflammations, as well as mental impairments over years. The exact causes of IBD are not known. Genetic factors as well as environmental influences are involved and cause the destruction of the homeostasis on the intestinal epithelial barrier, as well as the chronic activation of the local immune system through microbial components. In spite of significant efforts to develop new CED treatment approaches, so far there are only symptom-related treatments rather than causal and curative ones. More recent studies have shown that the aryl hydrocarbon receptor (AhR) might provide an approach for a causal therapy.
Therefore, repurposing libraries were used to identify AhR ligands which have already been classified as harmless in pre-clinical and clinical studies for other indications. Selected candidates were initially examined in simple in-vitro models with bone marrow-derived macrophages from mice for the induction of anti-inflammatory effects (interleukin(IL)-10 induction, IL-1 suppression). Afterwards, suitable candidates were tested in more complex in-vitro models (Transwell® and organoid models on the basis of human intestinal epithelial cells). In this context, the essential test parameters included the induction of certain tight-junction proteins and cytokine receptors. During this test phase, four candidates were identified as promising for the further development of active agents; two of which have already been examined in two candidates in two different in-vivo models (dextran sodium sulphate and bacteria-induced colitis in mice). However, since no significant therapeutic effect was achieved with these two candidates, two further candidates have been included in the in-vivo study and an additional candidate has been included in the second in-vitro testing stage.
Project partner
Fraunhofer CIMD, Fraunhofer IIS, Fraunhofer ITMP, Fraunhofer ISC
Funding
Fraunhofer CIMD
Human bones possess the remarkable feature to heal completely without forming fibrous scar tissue. However, different conditions may lead to a delayed, compromised or absent bone regeneration. Possible reasons could be a critical size fracture or systemic disease conditions, e.g. osteoporosis or type-2 diabetes mellitus (T2DM). In the latter case, tissue revascularization and differentiation of bone forming osteoblasts is often compromised. However, the extent of impaired healing capacities depends on each patient and until now, no diagnostic biomarker exists for prognosis of impaired bone healing before treatment begins. This often increases the burden of patients that suffer from non-healing fractures in standard therapies before a bone implant crafted from inert materials, autografts or allografts is applied. However, those materials are not optimal from different perspectives.
To this end, the SyMBoD projects aims to develop a digital platform for decision-making in treating patients with bone defects suffering from T2DM. This includes (ii) the identification of theranostic biomarkers and (ii) the modeling of individualized, patient- and fracture-specific scaffolds for fracture bridging. Therefore, different tissues (blood plasma and cells, bone tissue and exosomes) from both animal models and human biobanks will be screened in a multi-Omics approach to extract individual molecular profiles. Those profiles will be correlated to further clinical parameters and healing processes by AI-supported bioinformatic methods to stratify patients into risk groups and to identify theranostic biomarkers.
In parallel, bioresorbable polycarpolacton-based scaffolds will be optimized, based on multi-scaling modelling and iterative testing in animal models. This will allow for (i) optimizing biomechanical properties of the material at different size scales and (ii) developing computer models for the prediction of optimal individualized patient- and fracture-specific scaffolds.
Both, molecular and biomechanical models will be implemented into the platform and will guide clinicians (i) to identify risk patients based on selected prognostic biomarkers and (ii) to seamlessly create computer models of individualized scaffolds based on fracture imaging. Finally from those models, real bone implants can be manufactured in a GMP-compliant manner applying CAD-CAM 3-D printing techniques with biocompatible and resorbable materials breaking ground for personalized therapy in bone healing.
![BMBF_CMYK_Gef_M [Konvertiert]](/en/departments/leipzig-location/preclinical-development-and-validation/projects/jcr:content/contentPar/sectioncomponent_1564075601/sectionParsys/imagerow_copy/imageComponent1/image.img.jpg/1715948938243/BMBF-gefoerdert-2017-en.jpg)
Around the world, millions of people suffer from chronic illnesses which are a causal contributor in around 70 % of all deaths recorded. Often, the patients concerned receive recurring drug treatments over years; however, these only treat the symptoms, cause increasing side effects and, as a result, also involve mental strain for the patients. In view of an ageing population, chronic illnesses pose increasing socioeconomic challenges for society.
The ISOS consortium aims to develop the first bio-medical product for the on-site production and automatic administration of therapeutic agents. This product is based on probiotic, genetically modified bacteria (GMB) which produce the therapeutic molecules “on demand”, stimulated by the signals of the pathological environment within the patient. The GMB are embedded in a bio-reactor which is based on bio-materials, maintains the vitality of the bacteria and controls the release of the biologically produced active agent. This GMB ecosystem is to be designed so as to ensure that the bacteria cannot survive outside the encapsulated biological material in order to comply with future requirements regarding biological safety and legal requirements.
As a proof of concept, the ISOS consortium is working on the development of an implantable bioreactor based on GMB for treating exudative, age-related macular degeneration (AMD). This is characterised by the pathological growth of blood vessels in the retina which is caused by the abnormal production of the vascular endothelial growth factor (VEGF). At present, the only form of treatment is the repeated intraocular injection of anti-VEGF antibodies, which often creates psychological challenges for patients. Within the ISOS project, computer-supported models are intended to forecast VEGF-neutralising peptides and nanobodies which are then to be confirmed in experiments. Afterwards, probiotic bacteria will be genetically engineered to produce these anti-VEGF molecules and then implanted within the eye, embedded as a GMB bioreactor. This single implant and the continuous production of VEGF-neutralising molecules have the potential to replace the repeated injections of anti-VEGF antibodies.
The researchers from Fraunhofer IZI are materially involved in the experimental confirmation and characterisation of the interaction between the forecast anti-VEGF molecules and VEGF. To this end, bonding kinetics, mean inhibitory concentrations and molecular bonding sites are determined in order to select final molecules from the library of candidates. The project uses methods for surface plasmon resonance spectroscopy, in-vitro bio-assays and HDX mass spectrometry. The second phase of the project comprises the nanoLC-MS-based proteomic characterisation of transformed bacteria to confirm the continuous secretion of anti-VEGF molecules, as well as the vitality of the bacteria.

Periodontitis is an inflammatory disease of the periodontium that can lead to destruction of tissue and bone around the teeth. The disease is caused by bacteria that settle in plaque and gum pockets and trigger an inflammatory response. If left untreated, this leads to damage to the entire periodontium and even tooth loss. Various studies also show a direct link between periodontitis and other diseases such as cardiovascular disease and diabetes, as well as an increased risk of stroke.
PerioTrap Pharmaceuticals GmbH, a Fraunhofer IZI spin-off, assumes the preclinical development of novel periodontitis treatments as part of the Paropaste project. The basis of the novel treatment concept is the inhibition of an enzyme that occurs almost exclusively in the bacteria that cause periodontitis and regulates the production of various virulence factors there. By selectively inhibiting these factors, the pathogenic germs can be specifically suppressed and the natural microbiome preserved. The use of classical antibiotics, on the other hand, leads to growth inhibition of all oral germs, which carries the risk of rapid and stronger recolonization by the pathogens.
The aim of the project is to test appropriate drug candidates for their efficacy and safety, thus creating the prerequisite for a clinical trial for initial testing in humans. The collaborative partners will address various regulatory aspects, including active ingredient formulation, tolerability, efficacy and toxicity.
Fraunhofer IZI will contribute its expertise in the development and validation of bioanalytical methods for the comprehensive characterization of small molecule drugs. In addition, toxicity and safety are being investigated both in vitro and in animal models as part of a GLP study.
This project is sponsored by the German Federal Ministry of Education and Research as part of its “KMU-Innovativ Program” for small and medium-sized companies.
Partners
PerioTrap Pharmaceuticals GmbH; Skinomics GmbH; Fraunhofer IMWS
![BMBF_CMYK_Gef_M [Konvertiert]](/en/departments/leipzig-location/preclinical-development-and-validation/projects/jcr:content/contentPar/sectioncomponent_142278678/sectionParsys/imagerow_copy_copy/imageComponent1/image.img.jpg/1715946562923/BMBF-gefoerdert-2017-en.jpg)

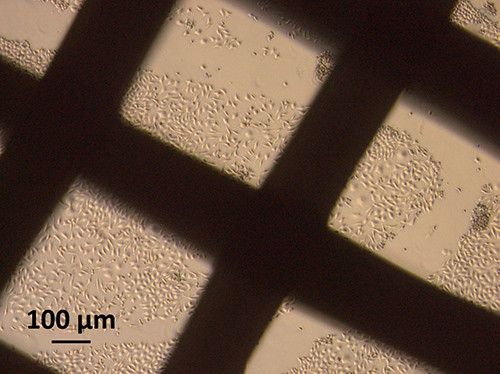
Breast cancer is the most common form of cancer among women around the globe, with therapy often involving the surgical removal of breast tissue. Following this kind of mastectomy, there are only a few options for reconstructing the breast. And these are not without their complications. The company BellaSeno GmbH has come up with an innovative solution strategy that presents an alternative to traditional implant products, e.g. silicone, to reconstruct the breast. By implanting a patient-specific scaffold structure made of bioresorbable polycaprolactone that is then filled with the patient‘s own body fat, the company’s approach to reconstruction would entail fewer complications. The final development stage of the implant as well as its manufacture and safety testing for approval as a medical device will be supported as part of an SAB project involving BellaSeno GmbH, GeSIM GmbH and Fraunhofer IZI.
Once BellaSeno GmbH has completed the final development stage, the scaffold structure will be manufactured using a 3D printing method under GMP conditions in the Department of GMP Cell and Gene Therapy. The implantation strategy and the functionality of the implant will then be evaluated in the large animal model. As the new implant is a risk class III medical device, preclinical and clinical trials have to be carried out in accordance with the German Medical Devices Act in order to ensure biological safety in patients. The preclinical safety trials will be conducted in Fraunhofer IZI’s GLP test facility based on DIN EN ISO 10993. The trials characterize and analyze the degradation products used in the resorbable scaffold structure and also test for any potential cytotoxicity in vitro and systemic toxicity in the mouse model. Degradation studies have already been conducted in order to characterize the implant in greater detail; these studies identified the individual degradation products that make up the resorbable implant. Furthermore, the cytotoxic potential of the scaffold structure is currently being investigated in vitro under DIN EN ISO 10993-5. This will be followed by tests on local effects following implantation as well as on systemic toxicity in the mouse.
The aim of the project is to build the foundations for approving an alternative kind of implant to improve the regeneration of breast tissue with few complications. The longer term goal is for the implant to be approved as a medical device and tested as part of a clinical trial.

