Department of Cell and Gene Therapy Development
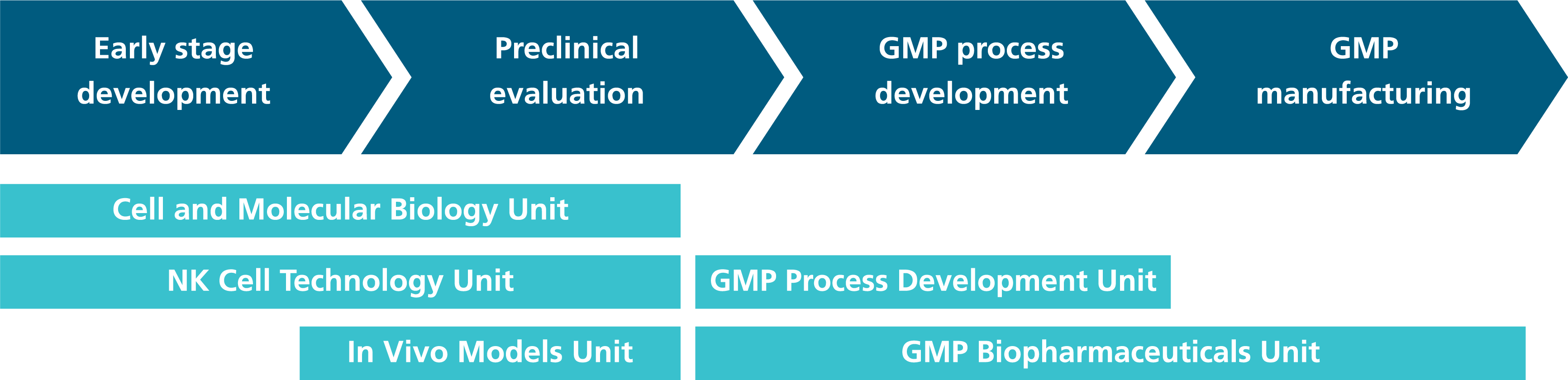
The Department of Cell and Gene Therapy Development researches and develops cell and gene therapy technologies and realizes the transfer of manufacturing processes from an experimental stage to GMP-compliant procedures.
The focus is on antigen-specific T cells, CAR T cells, CAR NK cells, dendritic cells, mesenchymal stromal cells and tissue engineering products.
The department's competencies, which build on each other, include research and development, preclinical evaluation and GMP process development for cell and gene therapies up to transfer into pharmaceutical manufacturing processes. Manufacturing parameters and quality controls can be tested and optimized flexibly and cost-efficiently.
New technologies (including digitalization, artificial intelligence, automation) as well as clinically relevant application aspects are considered at all stages of development.
In addition, biomolecules such as antibodies, proteins, enzymes and, in the future, viral vectors are produced in pharmaceutical quality in a separate GMP manufacturing unit.
After successful process optimization, investigational medicinal products can be produced by the Department of GMP Cell and Gene Therapy and further accompanied until approval.