Contact Press / Media
Dr. Anna Dünkel
Head of GMP Development Unit
Fraunhofer Institute for Cell Therapy and Immunology
Perlickstraße 1
04103 Leipzig, Germany
Phone +49 341 35536-3612


The aim of the project is to develop an integrated and automated manufacturing platform for the decentralized production of CAR-T cell therapeutics. The system, which can be placed in hospitals and medical centers, is intended to significantly reduce both the production times and the costs of manufacturing cell-based immunotherapeutics and thus expand access to these vital drugs.
To this end, an EU-backed consortium consisting of 18 industrial and academic partners is working together under the coordination of Fresenius. The basis for the project is a technology developed by Fresenius Kabi, which is being further developed as part of the project.
The Fraunhofer IZI is contributing its expertise in the development and production of CAR-T cell therapies and various other cell-based therapeutics and is taking on various tasks within the consortium. These include the testing and optimization of the point-of-care manufacturing platform and corresponding consumables as well as their validation against conventional manufacturing processes in the centralized laboratory. The Fraunhofer IZI is also involved in the development of studies and concepts focusing on the integration of the platform into clinical workflows and regulatory aspects.
Fraunhofer IESE is also involved, which is developing a digital twin of the technology that will be used for further optimization.
Funding
This project is supported by the Innovative Health Initiative Joint Undertaking and its members and industry partners, under grant agreement No 101194710.
Disclaimer
Funded by the European Union, the private members, and those contributing partners of the IHI JU. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the aforementioned parties. Neither of the aforementioned parties can be held responsible for them.
Partner
Fresenius SE & Co. KGaA (Koordination) (DE), Helios Hospital Berlin-Buch (DE), QS Instituto (ES), Fenwal Inc. (US), Cellix Ltd. (IE), Charles River (DE), Pro-Liance Global Solutions (DE), TQ Therapeutics (DE), Philips Electronics Nederland B.V. (NL), Fraunhofer IESE (DE), Helmholtz-Zentrum Dresden-Rossendorf (DE), Technical University of Denmark (DK), Frankfurt School of Finance & Management (DE), European Society for Blood & Marrow Transplantation (SP), Bar-Ilan University (IL), University of Glasgow (UK), University of Navarra (ES).
Novel cell and gene therapies, such as CAR-T cell therapy, are continuously expanding the treatment spectrum in cancer medicine. Impressive clinical successes have been demonstrated in recent years, particularly in the treatment of certain hematologic cancers. Initial treatment successes in individual patients with autoimmune diseases show that the medical potential is also enormous beyond the field of cancer medicine. However, as the triggers of autoimmune diseases differ significantly from those of oncological diseases, there is still extensive potential for research and development. To date, there are no curative treatment approaches for these diseases.
Most autoimmune diseases are triggered by self-reactive T cells, which mistakenly recognize healthy, endogenous structures as “foreign” and then attack them. The DepleTe project uses modern genome engineering to enable immune cells to recognize and destroy self-reactive T cells. The project is thus laying the foundations for innovative cell therapies that specifically combat the triggers of autoimmune diseases without impairing the functions of the healthy immune system.
On behalf of the University Medicine Göttingen, a GMP-compliant master cell bank is being developed and produced as the basis for a pharmaceutical manufacturing process for an AAV-based drug. For this purpose, a suitable cell line is first identified and established, which is adapted to the client's specific product. After successful expansion, the cells are comprehensively characterized to determine their identity, purity, vitality and biological activity. In parallel, comprehensive quality control is implemented.
All steps and results were carefully documented to ensure the traceability of the process. Finally, the master cell bank was approved by the quality assurance team, confirming GMP compliance. This structured approach ensures that the master cell bank meets the highest quality and safety standards.
The current protocols for the autologous ex-vivo gene and cell therapy comprise a work-, time- and cost-intensive supply chain. The patients’ cells are usually extracted in a specialized clinical center and delivered to a central production facility, where the cells are genetically modified, expanded and subjected to quality controls. After this, the cryopreserved cell product is returned to the hospital, where the treatment is carried out. At present, production protocols are being developed to significantly reduce production times and costs in order to cover the growing future demand and facilitate comprehensive access to cell and gene therapies.
The current methods for genetic engineering of therapeutic cell products have a number of limitations. These include the lack of long-term expression of transgenes, the risk of insertional oncogenesis and other harmful mutations of the recipient cells’ genome. Moreover, the production of genetic vectors of pharmaceutical quality involves considerable costs.
Non-viral gene transfer technologies constitute a promising option to make the production of genetically modified cells considerably more cost effective.
The simplest gene transfer vehicles known from nature are transposons. Genome integration using transposase enzymes turns transposons into unique non-viral vector systems, which can be used as tools for various applications in genetic engineering, including gene therapy. One of the best characterized transposons, the Sleeping Beauty transposon, offers great potential for the development of safe and commercially viable cell therapeutics. So far, however, the sub-optimal efficiency of genome integration in certain human cell types, the insufficiently controlled transposase expression in cells and the risk of insertional mutagenesis as a result of random genome-wide integration still constitute relevant limitations of the technology.
This project aims to improve the effectiveness and safety of the Sleeping Beauty transposon technology and prepare it for clinical development.
Chimeric antigen receptor (CAR) cell therapeutics are considered to be a groundbreaking innovation in cancer treatment. So far, they have only been approved for a few hematological types of cancer. However, numerous preclinical studies, as well as a growing number of clinical studies, have confirmed the technology’s enormous potential for other oncological indications, and beyond.
Today, approved CAR cell therapeutics are manufactured by means of a stable genetic modification of immune cells via viral transduction. But the viral generation of CAR immune cells is a very time-, material- and cost-intensive procedure and, for this reason, it is not suitable for extensive testing of new CAR designs using screening methods.
To remedy this, Fraunhofer IZI has developed a time- and cost-saving method based on mRNA transfection for the efficient generation of new CAR immune cells. This method permits high throughput screening for the fast identification of optimal candidates for new CAR cell therapeutics and functional testing for anti-tumor effectiveness. This will significantly speed up preclinical development.
The new method has now been patented and is available for partners from both academic and industrial research and can be easily and cost-effectively adapted to other tumor types or diseases, such as autoimmune diseases or fibroses.
This project aims to provide a technology platform to accelerate the preclinical development of CAR-modified immune cell therapies. The efforts will focus on types of cancer which are difficult to treat and on solid tumors.
The targeted combination of CAR modifications and gene editing aims to improve anti-tumor activity, tumor infiltration and longevity of T and NK cells and to overcome immunosuppressive factors in the tumor environment. To achieve this, the consortium will use a broad range of identified target molecules to generate CAR immune cell products, which will be developed and validated as part of this project.
Fraunhofer IZI is contributing its expertise in the development of CAR-modified T and NK cell therapies and in non-viral gene transfer technologies (Sleeping Beauty transposon). Another field of action comprises the development of pharmaceutical manufacturing processes for immune therapies as the basis for clinical testing.
Project partners
Frankfurt University Hospital (Coordination); Würzburg University Hospital (Coordination); Freiburg University Hospital

Fraunhofer IZI is a member of the European Rare Diseases Research Alliance (ERDERA).
This consortium (supported by the EU) aims to improve medical care for people living with a rare disease through coordinated research in the fields of the prevention, diagnosis and treatment of rare diseases.
As part of this, Fraunhofer IZI contributes its expertise in the process development and production of mRNA, LNP and AAV-based therapeutics. Its commitment focuses on the harmonization of development and production processes to accelerate the transfer of promising research results into clinical applications throughout Europe.
This project aims to develop standardized and scalable processes for novel gene transfer methods for cell and gene therapy manufacturing. These gene transfer methods include adeno-associated vectors (AAV) as a gene transfer construct for gene therapies as well as mRNA lipid nanoparticles (mRNA LNP) for cell therapies.
The development focuses on scalable and GMP-compliant manufacturing processes for adeno-associated vectors (AAV) which can be used, in particular, in gene replacement treatment. The cooperation with the industry partner Cytiva combines its technical resources and process know-how with the scientific and process-engineering expertise of Fraunhofer IZI.
Another focus is on the production of GMP-compliant manufacturing process for mRNA-based CAR-T cells aimed at CD19 for the treatment of serious autoimmune diseases. For this purpose, Fraunhofer IZI designs and produces mRNA molecules. After that, Cytiva, the cooperation partner, will provide support in encapsulating the mRNA in lipid nanoparticles, as well as in upscaling the CAR-T cell generation. Optimal encapsulation is necessary in order to transfer the mRNA into the T cells in a stable and precisely aimed manner. Then, the manipulated T cells can detect immune cells with the (CD 19) surface antigen and initiate a corresponding immune response. For the clinical use of such therapies, all manufacturing steps (from the mRNA design and encapsulation to the production of effector cells) have to fulfil pharmaceutical standards. Fraunhofer IZI is contributing its long-standing expertise in the development, optimization and evaluation of corresponding and relevant methods.
Partner
Cytiva

This project aims to produce a stable human cell line for the efficient, cost-effective and safe production of clinical test specimens of adeno-associated viral vectors (AAV). To this end, as a first step, a master cell bank will be established on the basis of HEK293 cells, which will be stably transfected with the help of lentiviral vectors. As a next step, a stable expression system for the AAV vector production is to be developed. In the framework of this project, the genetic information for serotype AAV2 and a vector design which carries an eGFP (enhanced green fluorescent protein) are to be introduced into the HEK293 cells, by way of example.
The cell line will fulfil the regulatory requirements of the Good Manufacturing Practice (GMP) under the EU-GMP Guideline and, therefore, it will be of sufficiently high quality for use as a source material for drugs.
As a result, a reliable and efficiently scalable system will be established in line with the pharmaceutical industry’s high quality requirements and will optimize the production of AAV vectors for use in gene therapy.
Immunotherapies based on T cells and natural killer cells (NK cells) are primarily produced from peripheral blood which is obtained from patients or donors through apheresis. To ensure that the apheresates can be provided in sufficient quantities for research and development, a standardized freezing process which should be as gentle as possible is required. In addition, the freezing process should also permit longer storage, as well as shipping of these starting products while assuring their quality. Therefore, this project aims to develop an optimized process for the separation and cryopreservation of leukapheresis products to permit fast and cost-effective access to this valuable starting material for the development of novel, cell-based medication.
To this end, fresh apheresates are prepared, divided into several batches and then cryopreserved using an optimized method at Fraunhofer IZI. The fresh apheresate and the defrosted products are then analyzed and compared in terms of their cellular parameters, such as cell count, vitality, composition and functionality, focusing on collecting comparative data regarding the phenotype and the fitness of the primary T and NK cells. In addition, the impact of cryopreservation on proliferative capacity and the cytotoxic activity of the immune cells relevant for treatment is examined.
Client
Haema AG
This project aims to develop innovative irradiation processes for the production of modern cell and gene therapeutics.
Low-energy electron irradiation (LEEI) is an irradiation method suitable for the efficient inactivation of pathogens (such as viruses and bacteria) and eukaryotic cells. This inactivation method is based on the destruction of the genetic information (nucleic acids).
The new method has been patented, and Fraunhofer IZI has a research prototype which is unique worldwide, and which can be used to develop this irradiation technology and adapt it to various applications.
The project will evaluate low-energy electron irradiation for two specific application scenarios: The first application scenario will include the irradiation of leukocytes as an alternative method in extracorporeal photophoresis. Under the current method, the cells are treated using ultraviolet radiation with the addition of a photosensitiser (a light-activated substance). This treatment is, e.g., used in graft-versus-host disease, the main complication after allogenic haematopoietic cell transplants. If low-energy electron irradiation is used, the addition of a photosensitizer (which involves side effects) is not necessary.
The second application addresses the production of NK cell-based immune therapeutics. Unlike cell therapeutics from T effector cells (such as CAR T cells), natural killer cells have to be co-cultivated in a complex process using feeder cells to achieve the clinically required quantities of therapeutic cells. If feeder cells are used in GMP production processes, their growth is usually inhibited using irradiation methods for safety reasons. The suitability of LEEI as an alternative inactivation method for feeder cells will be examined as part of this project.
Building on the initial successes of T cell-based cancer immunotherapies and expanding both their scope and variety of applications, another type of immune cell is receiving increasing attention in biomedical research: natural killer (NK) cells.
Unlike T cells, NK cells also lend themselves to allogeneic forms of therapy as they can be safely transferred between healthy donors and cancer patients. This facilitates standardizable and cost-effective stock production, which allows the products to be retrieved according to demand.
Before allogeneic NK cells can be employed as an efficient medicine, they first have to be genetically modified and equipped with new receptors that are able to recognize cancer cells. The strategic partnership between the researchers at Oslo University Hospital and the Fraunhofer Institute for Cell Therapy and Immunology focuses in part on modified T-cell receptors (TCR), which are able to recognize fragments of intracellular tumor antigens on HLA-I complexes. Compared with CAR (chimeric antigen receptor) T cells, which can only recognize surface antigens, this makes for a much broader spectrum of potential target antigens.
In order to translate pertinent research findings into clinical application as quickly as possible, process solutions for pharmaceutical production are directly considered and factored in at every stage of development. Fraunhofer institutes IZI and IPA (Institute for Manufacturing Engineering and Automation) are also contributing their experience in the fields of GMP process development and the development of automation solutions for the manufacture of cell therapeutics.

REANIMA aims to provide innovative therapies for heart regeneration. It is the first project in Europe to include results from fundamental research with the aim of translating these into medical applications. The knowledge gained from animal models is to be comprehensively analysed to develop new, regenerative therapies to treat congestive heart failure. This project is funded by the EU Horizon 2020 programme. Fraunhofer IZI is a member of the project consortium which brings together twelve European partners.
Project coordination
Centro Nacional de Investigaciones Cardiovasculares (CNIC)
Grant Agreement No
874764

Cell and gene therapies are innovative treatment methods facilitating curative approaches to severe, previously incurable diseases. This includes therapies using genetically modified cells as advanced medicinal products (ATMP). In CAR T cell therapy, the patient’s own T cells are modified with chimeric antigen receptors (CAR). Both the approved CAR T cells and the majority of new CAR T cells currently being developed are based on the stable genetic engineering modification of the patient’s own cells with the help of viral vectors. However, since the CAR T cell therapy is still a very new method, long-term effects have not been fully studied. Furthermore, persistent CAR T cells partly cause severe side effects. The temporary modification of cells using a messenger RNA (mRNA) coding for the CAR protein constitutes an alternative to the stable version.
The competence platform aims to develop transient CAR cell therapeutics to treat immune-mediated diseases. For this purpose, new mRNA technologies and nano-transporter systems will be developed. As a result, an establishment project is to generate CAR T cells against activated fibroblasts. Human 3D cell culture and tissue models of fibrosis as well as a novel imaging platform will be used for functional testing. Another goal is to transfer this technology to natural killer (NK) cells to develop donor-independent CAR cell therapies.
Moreover, the platform will be used to develop mRNA-based CAR cell therapeutics with a higher safety profile. This results in a transient ATMP approach to the treatment of fibrotic diseases. To cover the future demand for CAR cell therapies, the transition from autologous products (using the patient’s own cells) to allogenic (genetically different) products is supported so that one product batch can be used to treat as many patients as possible.
If the establishment project is successful, further ex vivo models of fibrotic tissues are to be used for CAR cell testing in cooperation with Fraunhofer ITEM. Concurrently, the platform is to be expanded with other cell-therapeutic effects (e.g. T cell receptor-modified cells) and other target indications (e.g. arthrosis) in the medium term.
The launch of the first programmed killer cells (chimeric antigen receptor (CAR)-carrying T cells; product name ”Kymriah” from Novartis AG) has significantly expanded the therapeutic options for blood cancer patients. However, the use of CAR-modified T cells, due to their biological properties, remains below expectations, especially in the treatment of solid tumors. This is mainly due to the fact that the therapeutic cells are often not able to penetrate into the tumor mass. In this context, it is known that the tumor environment (the so-called microenvironment) inhibits the activity of programmed killer cells. To actively address these challenges, the suitability of different starting cells for developing new cell and gene therapeutics will be tested. In this project, CAR macrophages will be used to generate and implement a new cellular therapeutic approach against solid tumors that have been difficult to treat so far. For this purpose, macrophages are isolated from human donor material and subsequently equipped with chimeric antigen receptors (CAR) directed against prominent tumor antigens. The ability of the CAR macrophages to target tumor cells is expected to be maximized by inducing and locally releasing type I interferons (type1 IFNe). In addition, macrophages are expected to reprogram the tumorigenic milieu of the solid tumor into an anti-tumorigenic milieu to force tumor growth arrest while sensitizing tumor cells to standard therapies. According to their biological function, macrophages can further: 1. actively phagocytize tumor cells and 2. present tumor-specific antigens, which in turn activate other immune cells to fight the tumor.
The use of CAR macrophages can greatly expand therapeutic options for various types of tumors. Unlike expensive, patient-specific cell therapeutics, macrophages can also be used and applied from foreign donors (as an allogeneic product). In particular, transport routes and times can be reduced and the availability of therapies for affected patients can be increased enormously.
The project is characterized by its translational character, since not only the conceptual and technical feasibility of CAR macrophages in a biological context will be addressed, but also a standardization of CAR macrophage production with the help of appropriate protocols will be secured and described.
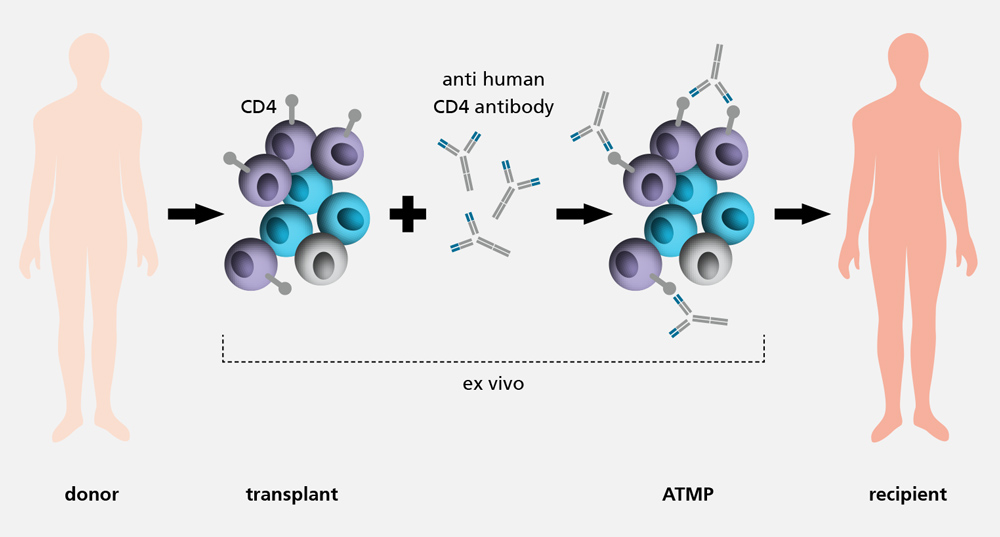
With an incidence of 30 to 40 percent, the graft-versus-host disease (GvHD) is one of the main complications after an allogenic haematopoietic cell transplant. Conventional treatment methods aim for an unspecific suppression of the entire immune system, which can significantly increase the risk of infections and relapses. Moreover, the long-term success to be expected might be low and associated with both hepato- and nephrotoxic side effects. As a result, the development of less straining alternative treatments is urgently needed.
The GMP process development / ATMP design department is developing protocols and methods to prepare the production of the advanced therapy medicinal product (ATMP) Palintra® to prevent GvHD under GMP conditions. Pre-incubation of a haematopoietic cell transplant with an anti-human CD4 antibody reduces undesired immune responses against the host tissue after transplantation. However, the graft-versus-tumour (GvL) effect which protects against relapses is maintained.
As part of the pre-clinical development phase, cell-based functional assays are established. These potency assays can measure the function of the immunotolerance-inducing, anti-human CD-4 antibody in vitro for the first time ever. Moreover, next generation sequencing is to be used to detect changes in the transcriptome of T cells and to draw conclusions regarding the molecular effect of the antibody. Additionally, the treatment efficiency of Palintra® in GvHD prevention is examined in vivo and compared with conventional therapies.
In addition to fulfilling official pre-clinical requirements, the experiments listed above can generate new insights into immunological processes in inducing immunotolerance and into GvHD. These models and insights are particularly important not only for haematopoietic cell transplants, e.g., in leukaemia treatment but also for stem cell transplants for other indications (e.g. autoimmune diseases).
The measure is co-financed with tax funds on the basis of the budget approved by the Saxon State Parliament.

Cell and gene therapeutics, so-called advanced therapy medicinal products (ATMPs), have a very high therapeutic potential. In hematology and oncology CAR-T cell therapy has been used in Germany, for example, since 2018. However, complex logistics processes from centralized production sites and inflexible manufacturing and application schemes make the production of these cell therapeutics very time and cost intensive. In the EU project "AIDPATH" (Artificial Intelligence-driven, Decentralized Production for Advanced Therapies in the Hospital), project partners from industry and research are now working on the development of an automated and intelligent facility capable of producing targeted and patient-specific cell therapy directly at the point of treatment, i.e. in the hospital. In addition, the project addresses the integration of the facility into the hospital environment, taking into account logistics processes as well as data management and data security.
Fraunhofer IZI is contributing its expertise to the project, particularly in the automation of manufacturing processes and plant networking. The main site in Leipzig has long been a central manufacturing and development site for a CAR-T cell therapeutic used to treat certain forms of blood cancer.
The "AIDPATH" project, which started in January 2021, is funded for four years under the European Union's Horizon 2020 framework program for research and innovation under grant number 101016909.
AIDPATH project consortium

Genetically engineered immune cells are revolutionizing cancer medicine. In recent years (from 2017 / 2018), the CAR-T cell treatment has established itself as an important treatment option for certain forms of leukemia and lymphoma.
In all CAR-T cell products which have been approved so far, the immune cells are genetically modified using modified viruses. These are then used as transport vehicles to permanently integrate the genes for the therapeutically relevant CAR receptor in the target cell’s genome.
In order to further develop this technology, Fraunhofer IZI researchers are evaluating alternative methods for genetically engineering immune cells with the aim of enhancing the safety and efficiency of treatment and developing further fields of application, e.g. in autoimmune disorders.
The direct transfer of messenger RNA (mRNA) as a template for producing therapeutic protein molecules in target cells constitutes one of the most promising alternatives to viral gene modification. Under this approach, transporting mRNA to the target site in the body constitutes the biggest obstacle to clinical application. To overcome this challenge, the mRNA delivery via nano-carriers has been optimized to transport the “securely packed” mRNA safely and efficiently. The mRNA is then released in the interior of the cells and the production of the therapeutic molecules begins.
In addition to functionality and optimal composition, initial safety-relevant toxicity testing was carried out. The results obtained now form the basis for the next step of development – a proof-of-concept study and safety assessment in an animal model. The project is sponsored by the Federal Ministry of Education and Research.
Partner
Fraunhofer Center for Applied Nanotechnology CAN
![BMBF_CMYK_Gef_M [Konvertiert]](/en/departments/leipzig-location/cell-and-gene-therapy-development/projects/jcr:content/contentPar/sectioncomponent_285/sectionParsys/imagerow_copy/imageComponent1/image.img.jpg/1716555642258/BMBF-gefoerdert-2017-en.jpg)
With an incidence of 30–60 percent, graft-versus-host disease (GvHD) is one of the main complications to follow allogeneic hematopoietic cell transplantation. Conventional treatment methods target a nonspecific suppression of the immune system, which can significantly increase the risk of infection and relapse. It is therefore all the more important that new drugs and therapeutic approaches are developed which, in the best case, maintain the function of a patient’s immune system while reducing adverse effects.
For several years now, extracellular vesicles (EVs) have been at the center of various research approaches relating to immune-mediated inflammatory diseases. Alongside a variety of diagnostic applications, anti-inflammatory and immunomodulatory effects are of particular interest to researchers. Almost every cell secretes EVs and a high number of cells resorb them. They thus play a fundamental role in intercellular communication and assume an important role in preserving physiological balance and in the pathogenesis of different diseases.
Measuring some 50 to 2000nm in size, the vesicles transport an abundance of biomolecules (including proteins, nucleic acids, lipids and metabolites). Immunomodulatory effects were able to be observed in various in vitro and in vivo studies, for example through EVs from stem cells.
On behalf of the company Lysatpharma GmbH (Eisenberg), whose technological focus lies in the field of regenerative medicine and the development of novel immunotherapies based on EVs, Fraunhofer IZI is evaluating the preventive and therapeutic potential of EVs in an in vivo GvHD model (mouse). Lysatpharma GmbH received support to carry out this preclinical research and product development through an economic development grant awarded to individual businesses by the Free State of Thuringia (project number 2019 FE 0152 (EFRE)).
Immunotherapies based on T cells and natural killer cells (NK cells) are primarily produced from peripheral blood which is obtained from patients or donors through apheresis. To ensure that the apheresates can be provided in sufficient quantities for research and development, a standardized freezing process which should be as gentle as possible is required. In addition, the freezing process should also permit longer storage, as well as shipping of these starting products while assuring their quality. Therefore, this project aims to develop an optimized process for the separation and cryopreservation of leukapheresis products to permit fast and cost-effective access to this valuable starting material for the development of novel, cell-based medication.
To this end, fresh apheresates are prepared, divided into several batches and then cryopreserved using an optimized method at Fraunhofer IZI. The fresh apheresate and the defrosted products are then analyzed and compared in terms of their cellular parameters, such as cell count, vitality, composition and functionality, focusing on collecting comparative data regarding the phenotype and the fitness of the primary T and NK cells. In addition, the impact of cryopreservation on proliferative capacity and the cytotoxic activity of the immune cells relevant for treatment is examined.
Client
Haema AG
This project aims to develop innovative irradiation processes for the production of modern cell and gene therapeutics.
Low-energy electron irradiation (LEEI) is an irradiation method suitable for the efficient inactivation of pathogens (such as viruses and bacteria) and eukaryotic cells. This inactivation method is based on the destruction of the genetic information (nucleic acids).
The new method has been patented, and Fraunhofer IZI has a research prototype which is unique worldwide, and which can be used to develop this irradiation technology and adapt it to various applications.
The project will evaluate low-energy electron irradiation for two specific application scenarios: The first application scenario will include the irradiation of leukocytes as an alternative method in extracorporeal photophoresis. Under the current method, the cells are treated using ultraviolet radiation with the addition of a photosensitiser (a light-activated substance). This treatment is, e.g., used in graft-versus-host disease, the main complication after allogenic haematopoietic cell transplants. If low-energy electron irradiation is used, the addition of a photosensitizer (which involves side effects) is not necessary.
The second application addresses the production of NK cell-based immune therapeutics. Unlike cell therapeutics from T effector cells (such as CAR T cells), natural killer cells have to be co-cultivated in a complex process using feeder cells to achieve the clinically required quantities of therapeutic cells. If feeder cells are used in GMP production processes, their growth is usually inhibited using irradiation methods for safety reasons. The suitability of LEEI as an alternative inactivation method for feeder cells will be examined as part of this project.
The launch of CAR-T cell therapy to treat various cancers constitutes an important milestone for the use of cellular immunotherapy in oncology.
Apart from the high hopes that this promising treatment option will become available for various types of cancer as soon as possible, the increasing numbers of patients, in turn, are also connected with various challenges. One of these is that CAR-T cell products have to be tailored to the individual patient in a complex production process, resulting in limited availability and high treatment costs.
Therefore, international research efforts are focusing on alternative immunotherapies, in addition to optimized production processes.
Natural killer cells (NK cells) are considered a promising resource to optimize the cost-efficiency and availability of cancer immunotherapies. CAR-NK cells are genetically engineered according to the same principle as CAR-T cells to enable them to find and destroy tumor cells. Unlike T cells, NK cells are not immunogenic. This means, they can also be transferred from healthy donors to patients without triggering immunological rejection. As a result, NK and CAR-NK cell products can be produced more cost efficiently and on a larger scale.
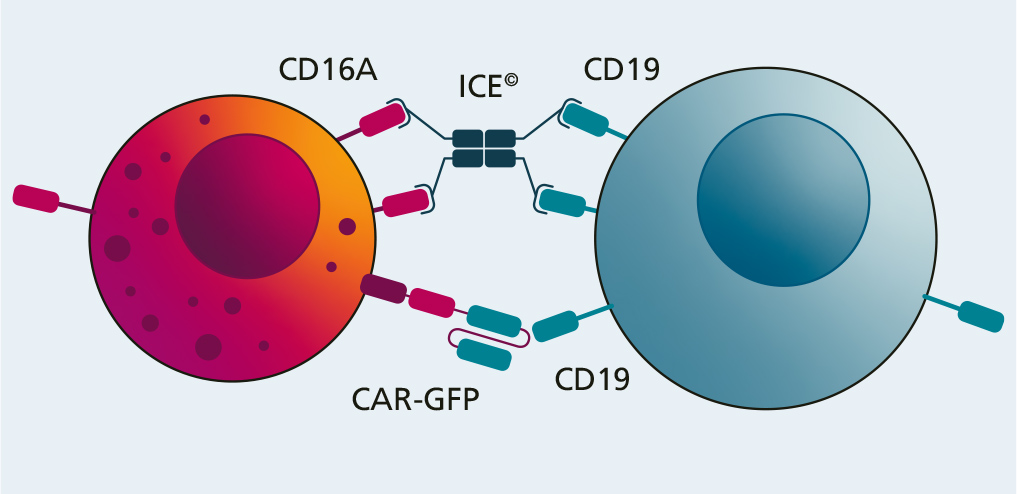
Fraunhofer IZI has examined the potential of a combination treatment comprising NK cell products and so-called innate cell engagers (ICE) on behalf of Affimed GmbH (Mannheim).
These ICE molecules attach to both NK cells and tumor cells by binding to the CD16A receptor on the immune cells and a specific antigen (CD19) on the tumor cells. As soon as this bridge is created, the immune cell is activated and destroys the tumor cell.
In this project, the antitumor effectiveness of two combination treatments (NK cells and CAR-NK cells each combined with ICE) was examined in various in vitro experiments compared with the simple CAR-NK cell treatment. This showed that the cytotoxic potential of the combination treatment is superior to the simple version. Apart from this, a significant difference was not observed between the two combinations (NK cell + ICE vs. CAR-NK cell + ICE). The use of ICE in combination with an NK cell treatment has the potential for successful cancer immunotherapy. However, this potential now has to be examined in further pre-clinical and clinical studies.
The aim of the project is the GMP-compliant production of a monoclonal antibody for the clinical trial for the prevention of Graft-versus-Host-Disease (GvHD) in allogeneic stem cell transplantation.
The project includes the following work packages:
Even today, there is frequently no cure for glaucoma, an eye disease. Therefore, research efforts to find new treatments must be accelerated. In many cases, current treatments and surgical methods are not effective, show little success and, frequently, are not well tolerated. Therefore, new treatments for the continuously increasing number of patients suffering from glaucoma are needed – in particular, by ensuring the permanent reduction of intraocular pressure. To achieve this effect, the use of innovative molecules is aimed at stabilizing the vascular endothelium.
This strategy is based on addressing a reversible small molecule inhibitor (vascular endothelial protein tyrosine phosphatase (VE-PTP)). A specifically developed fusion protein with a reinforced modulating effect on the signal path (referred to above) is intended to repair defective Schlemm canals in the eye - which leads to increased liquid flow. As a result, intraocular pressure would be reduced, which would, in principle, cure glaucoma.
Moreover, this therapeutic principle is to be evaluated in the context of SARS-COV-2 infections. This infection frequently involves damage to the endothelium – particularly in lung tissue. A stabilization of the endothelium would, therefore, be very valuable here as well.
For this reason, this project aims to establish a GMP-compliant production process (in particular as regards cell lines and master cell banks, as well as upstream and downstream process development) for the fusion protein. This includes the necessary quality control at Fraunhofer IZI so as to ensure the efficient and effective clinical application of the biomolecule.