Department of Cellular Immunotherapy
The Department of Cellular Immuntherapy offers broad expertise to support the development, manufacturing, and application of CAR immune cells. The team specializes in the genetic modification of T, NK, and macrophages using viral and non-viral gene transfer methods, as well as their functional and phenotypic characterization.
The following services are available upon individual agreement:
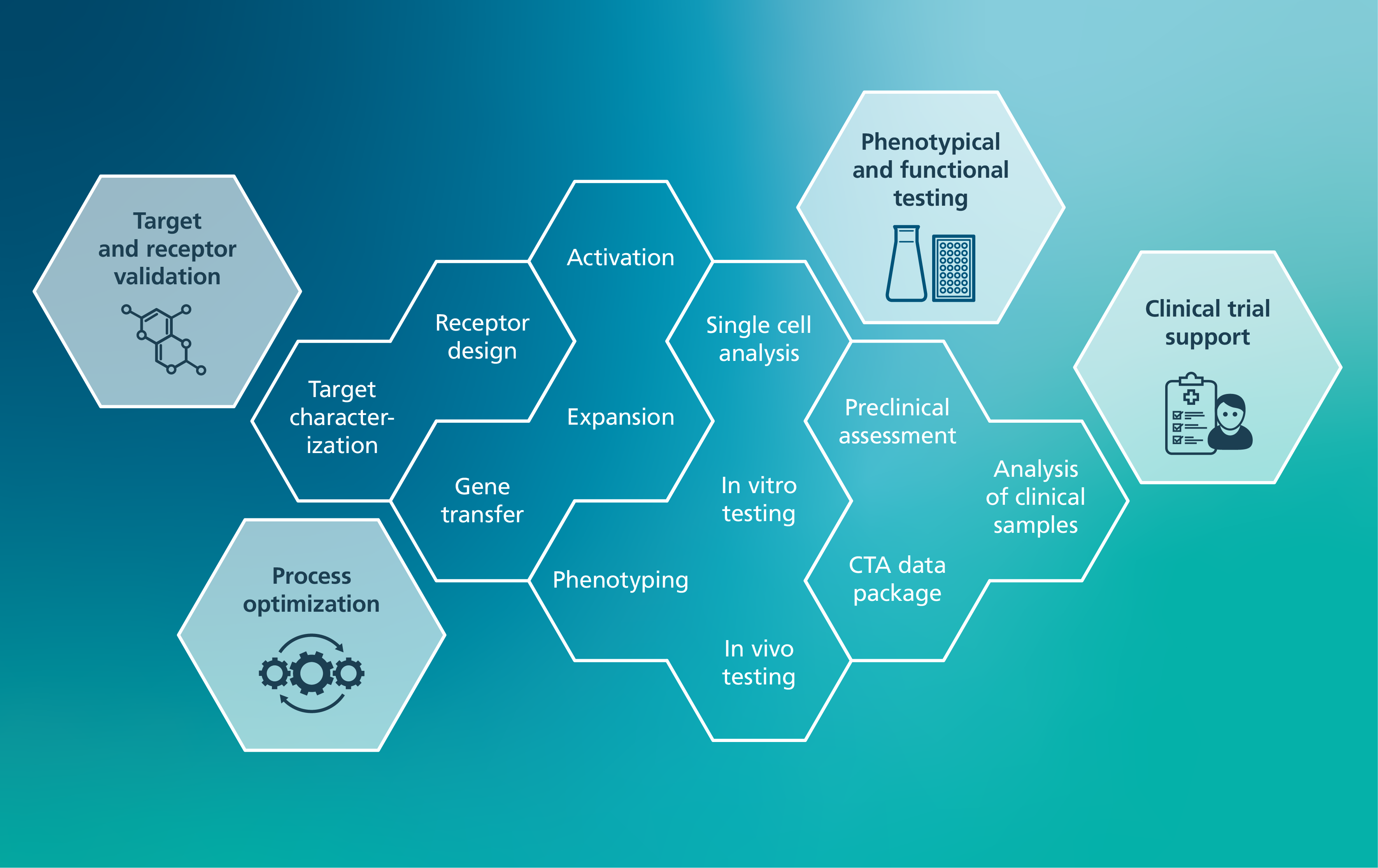
Target & receptor validation
The team supports you in tackling cancer and non-malignant diseases by developing optimized chimeric antigen receptors (CARs) tailored to your specific needs. Leveraging the extensive experience and proprietary CAR features, the group assists in target validation and CAR design to create receptors that ensure precise and effective target recognition.
This may include
- Characterization of target protein expression on malignant cells
- Optimization of CAR constructs using proprietary technologies
Process optimization
The transition of your reagents from development to scientific application is supported through process optimization in immune cell manufacturing. By leveraging the team’s expertise in protocol refinement, reagents and techniques are benchmarked against state-of-the-art manufacturing standards for immunotherapy.
This may include
- Activation reagents
- Gene transfer techniques, including packaging systems (e.g. LNPs/PNPs, viral systems) as well as integration tools (Transposases, e.g. Sleeping beauty)
- Cell-penetrating methods, such as electroporation, mechanoporation or squeezing
- New media formulation
- New culturing devices (bioreactors, culture vessels)
- Cytokines for supplementation during cell culture
Phenotypical & functional testing
The development of CAR constructs is supported through comprehensive phenotypic and functional testing to evaluate efficacy and specificity. Validated protocols, standardized operating procedures (SOPs), and established models ensure high-quality, reliable results. Services include detailed phenotypic characterization of immune cells at the protein level to facilitate a deeper understanding and optimization of their properties. In addition, functional analyses of CAR and immune cell effector mechanisms are conducted: Ranging from benchmarking final cell products to multi-assay profiling of individual immune cells. These evaluations are critical for assessing the therapeutic potential and safety of CAR immune cell products.
This may include
- Multiparameter phenotypic characterization of immune cells (activation, differentiation, exhaustion) via flow cytometry
- Tumor lysis of primary tumors and cell lines (Luciferin/flow cytometry/Incucyte)
- Cytokine secretion analysis (single cytokines or multiplex panels)
- Proliferation assays
- RNA Profiling (String technology)
- Single-cell analysis using the Beacon Select platform (by Bruker)
Clinical trial support
The team supports your clinical trial with expertise gained from investigator-initiated ATMP studies. Whether you're setting up and running a new trial or validating your technology for the analysis of clinical samples, expert support is available at every stage of the process.
This may include
- Generation of a preclinical data package (in vitro) to support clinical trial applications (e.g. for the Investigator’s Brochure)
- Analysis of clinical samples, including:
- Detection of ATMPs in blood via flow cytometry
- Quantification of serum cytokines
Other services
Device validation
The branch site enables biotech and pharmaceutical companies, as well as life science partners, to thoroughly test and validate devices used in the development of cellular immunotherapies. Standardized, clinically validated manufacturing protocols serve as a benchmark to objectively assess the performance and reliability of new technologies. Validated standards ensure the highest quality and safety requirements: An essential foundation for the successful development of innovative immunotherapies.
Testing & optimization of reagents for immune cell manufacturing
Novel reagents and techniques for CAR immune cell production are evaluated using standardized protocols derived from clinical immunotherapy manufacturing. An application note is collaboratively developed to support the promotion of your products.
This may include
- Comparative testing of activation and culture reagents (e.g. cytokines, media, stimulants)
- Support in developing and implementing alternative cell culture systems to improve process efficiency and sustainability