In vitro pharmacology
Cell and tissue technologies – Substance characterisation in vitro (GLP / non-GLP)
Permanent and primary cell models
The working group has long-standing expertise in experimental research using permanent mammalian cell lines and primary cultures of the central nervous system. Work under safety level 2 conditions is possible.

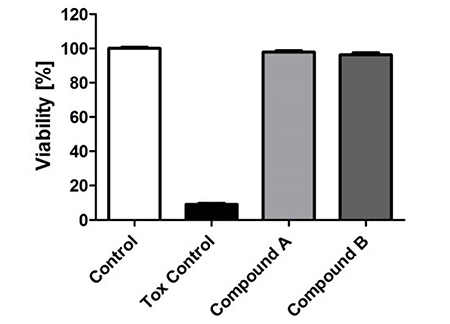
Toxicity
Cytotoxic and proliferative effects of new drugs are examined on permanent mammalian cell lines. >100 different mammalian cell lines are available for this. Because of their overarching importance for the metabolisation of drugs, toxicity is, e.g., tested on permanent human hepatocytes.
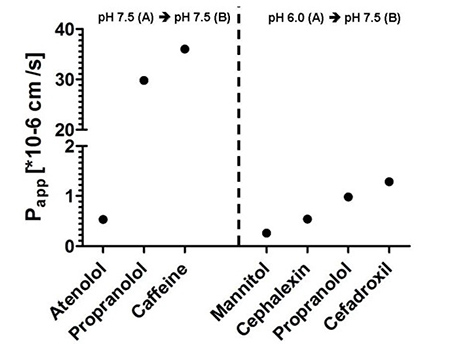
Transcellular transport
To assess the bio-availability in animal models, a cell-culture assay is available to study the transport of small molecules via an epithelial barrier. The cell model used consists of CaCo-2 cells, epithelial cells of a human colorectal adenocarcinoma. The analysis is carried out using LC-MS. The established apparent permeability coefficient (Papp) can be used to assess bio-availability.