Species
Mouse
Fields of application
EAE is induced by a single s.c. application of an emulsion containing Freund’s adjuvant, Proteolipidprotein 139-151(S) and desiccated Mycobacterium tuberculosis. On a clinical scale ranging from 0 to 5 EAE symptoms are assessed and body weight is monitored in a daily manner for at least thirty days.
The model can be used for the following fields of application:
- Pharmacodynamics and pharmacokinetics
- (Patho)-physiological processes
- Therapeutic efficacy
- Proof of concept
Endpoints / outcome parameter
- Disease severity: Level of disease (mean maximum clinical score, mean and median clinical score), extent of disease (total score, area under the curve), incidence, mean day of onset (in vivo)
- Body weight (in vivo)
- Central nervous system demyelination (ex vivo)
- Infiltration of immune cells into nervous tissue (ex vivo)
- Cytokine production
Readout parameter
- Clinical score
- Body weight
- Histology (Luxol fast blue and various classical histological stains)
- Immunohistochemistry
- ELISA, qRT-PCR
Quality management and validation
- Controls
- Randomisation
- Allocation concealment after immunization
- Blinded data collection and analysis
- Biometric expertise
- Internal quality management
Weblink
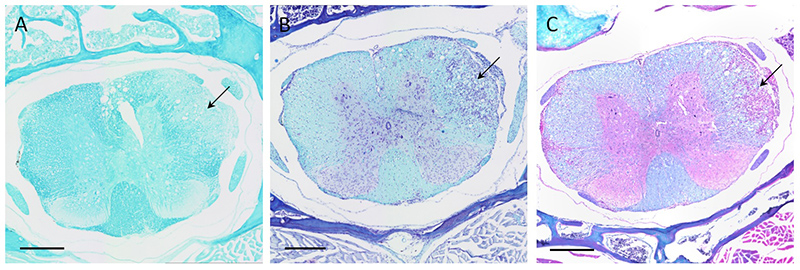
Serial coronal sections of the thoracic spinal cord from a mouse with a clinical score of 2.25. A: Luxol Fast Blue (LFB) stain. B: LFB + cresyl violet stain. C: LFB + hematoxylin and eosin stain. Blue = myelin, violet = cell nuclei, pink = cytoplasm. Arrow: demyelination in the white matter and infiltration of cells. Magnification: 10x. Scale bar: 300 µm.