Vaccine Technologies
ZOE – "Zoonosis Emergence accros Degraded and Restored Forest Ecosystems"
This project investigates the influence of changes in land use and a loss of biodiversity to the transmission of pathogens between animals and humans.
Zoonoses are diseases that can be transmitted from animals to humans. Infection can occur through direct contact with animals, through contaminated food or through vectors such as ticks and mosquitoes. Humans play a decisive role in the development and spread of zoonoses. Agriculture and livestock farming, as well as the trade and consumption of wild animals, create environmental situations in which pathogens can easily be transmitted between animals and humans. Added to this are interventions in natural habitats, such as the clearing of forests to make room for livestock or plantations, or the expansion of urban areas.
One of the aims of the project is to carry out a detailed mapping of biodiversity in forest areas in which humans have intervened to varying degrees. To this end, researchers in Guatemala, Costa Rica, Slovenia and Slovakia will study original forests as well as deforested and renaturalized areas. In order to determine the prevailing land use and biodiversity, the nature of the landscape and the animal and plant species will be recorded using satellite images and also directly on site. In addition, the scientists want to determine how many potentially dangerous microorganisms are circulating in the ecosystem by testing rodents, ticks and mosquitoes - as frequent carriers of zoonotic pathogens - for the presence of various bacteria and viruses using modern sequencing techniques.
The Fraunhofer IZI contributes its expertise in the field of virology and will develop assays to screen the samples from the study areas for all important groups of zoonotic pathogens. The samples mainly comprise blood samples from animals from the forest areas under investigation. In addition, blood samples from people living in the vicinity will be examined in order to find out how many of the zoonotic pathogens have already been transmitted.
Partners
Charité – Universitätsmedizin Berlin, Germany; Leibniz University Hannover, Germany; Biomedical Research Center of the Slovak Academy of Sciences, Slovakia; Universidad del Valle de Guatemala, Guatemala; University of Vienna, Austria; University of Ljubljana, Slovenia; University of Potsdam, Germany; Pikado B.V., Netherlands; University of Costa Rica, Costa Rica; University of A Coruña, Spain; Aix-Marseille University, France; Protisvalor, France; National Autonomous University of Mexico, Mexico; Centro de Investigación y de Estudios Avanzados, Mexico; Wildlife Conservation Society, USA
Funding
The research consortium “ZOE – Zoonosis Emergence accros Degraded and Restored Forest Ecosystems” is being funded by the European Union as part of “Horizon Europe”, the EU Framework Program for Research and Innovation, with around four million euros over four years.

Inactivation of therapeutic immune cells by low-energy electron irradiation
This project aims to develop innovative irradiation processes for the production of modern cell and gene therapeutics.
Low-energy electron irradiation (LEEI) is an irradiation method suitable for the efficient inactivation of pathogens (such as viruses and bacteria) and eukaryotic cells. This inactivation method is based on the destruction of the genetic information (nucleic acids).
The new method has been patented, and Fraunhofer IZI has a research prototype which is unique worldwide, and which can be used to develop this irradiation technology and adapt it to various applications.
The project will evaluate low-energy electron irradiation for two specific application scenarios: The first application scenario will include the irradiation of leukocytes as an alternative method in extracorporeal photophoresis. Under the current method, the cells are treated using ultraviolet radiation with the addition of a photosensitiser (a light-activated substance). This treatment is, e.g., used in graft-versus-host disease, the main complication after allogenic haematopoietic cell transplants. If low-energy electron irradiation is used, the addition of a photosensitizer (which involves side effects) is not necessary.
The second application addresses the production of NK cell-based immune therapeutics. Unlike cell therapeutics from T effector cells (such as CAR T cells), natural killer cells have to be co-cultivated in a complex process using feeder cells to achieve the clinically required quantities of therapeutic cells. If feeder cells are used in GMP production processes, their growth is usually inhibited using irradiation methods for safety reasons. The suitability of LEEI as an alternative inactivation method for feeder cells will be examined as part of this project.
COX-SAVE – Development of a safe Q fever vaccine for ruminants using electron-beam-inactivated Coxiella
Coxiellosis, also known as Q fever, is a global problem in breeding cattle and other ruminants. The disease is caused by the highly infectious Coxiella burnetii bacterium and, in addition to a reduced milk yield, it causes a wide range of reproduction issues, including miscarriages and the birth of weak calves.
This project aims to develop an inactivated vaccine exceeding existing options in terms of effectiveness and tolerability profile. As a result of the improvement and optimisation of existing vaccination strategies, morbidity as well as the unspecific use of antibiotics and other drugs are reduced and the general performance of livestock is preserved, in addition to their health. As Q fever is a zoonotic disease, in addition to the improvement of animal health, this also helps to lower the risk of infection of humans through the reduced excretion of Coxiella in livestock.
The development of the novel inactivated vaccine is based on a technology for inactivating pathogens using low-energy electron irradiation, which was developed by Fraunhofer. The irradiation of the pathogens with low-energy electron beams damages the genetic information (nucleic acid) inside the pathogen; in contrast, the proteins and antigens on the surface remain largely intact for use in vaccines, while preventing the further reproduction of the pathogen. This provides the option for a more specific immune response and, hence, an improved vaccine. Moreover, the use of the Nine Mile Phase II strain of Coxiella burnetii ensures the faster and more cost-effective inactivation of the pathogen at lower biological safety levels (BSL 2 instead of BSL 3) compared with other available vaccines.
In addition to the technology for inactivating the pathogens, as part of the project, Fraunhofer IZI also contributes its infection biology expertise to the analysis, characterisation and quality assurance of the vaccine candidates.
Project partners
Stiftung Tierärztliche Hochschule Hannover; Federal Research Institute for Animal Health, Friedrich-Loeffler-Institut (FLI); Wirtschaftsgenossenschaft deutscher Tierärzte eG
Further information
Development of a vaccine candidate against the West Nile virus
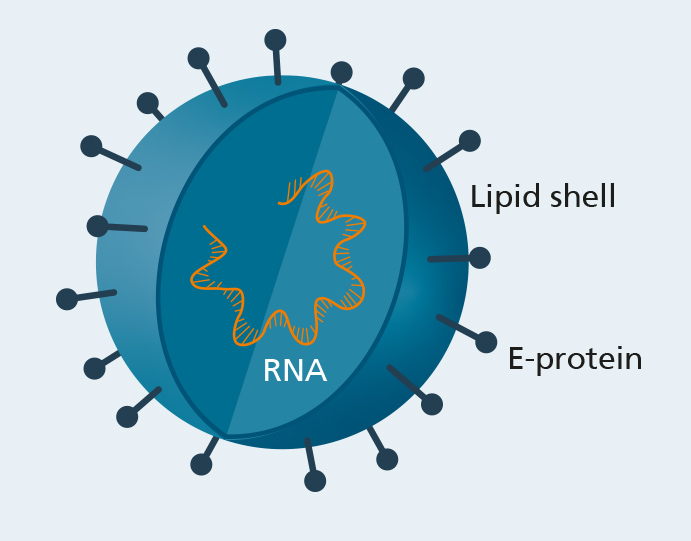
The West Nile virus (WNV) is a zoonotic flavivirus spread by mosquitoes. The virus primarily circulates among birds, but can also be transmitted to mammals, like humans. Even though, in most cases, an infection only involves mild, cold-like symptoms, it can also cause severe neurological problems, in particular, in older or immunocompromised patients. So far, a human vaccine against the West Nile virus is not available.
The envelope protein (E) is the most important target of neutralizing antibodies and, for this reason, it is of fundamental importance for vaccine development. It is located on the surface of the virus and has a central role at various points of the viral life cycle, especially, when the virus enters the host cells.
The close genetic relationship between WNV and other flaviviruses (such as the Zika virus or the Dengue virus) means that the E proteins are very similar in their sequence and structure and forms a particular challenge in the development of suitable vaccine candidates. In the case of an infection with other flaviviruses, the antibodies formed as a result of the immunization can lead to cross-reactions, which might amplify the infection and are associated with more severe courses of the disease. This is particularly difficult in regions in which several flaviviruses coexist.
Therefore, the aim is to develop improved protein- and mRNA-based vaccine candidates while avoiding cross-reactions with similar viruses.
Most cross-reactive antibodies bind the E protein in an area which is almost identical in most other flaviviruses, the so-called fusion loop. Therefore, a possible solution for immunization comprises the use of antigens in which this area has either been mutated or eliminated altogether. It was proven in an animal model that corresponding antigens induce a largely protective immune response. An analysis of the neutralizing antibodies showed a significantly reduced cross-reactivity with other flaviviruses, compared to the wild-type protein.
Prevention measures to break chains of transmission

Privacy warning
With the click on the play button an external video from www.youtube.com is loaded and started. Your data is possible transferred and stored to third party. Do not start the video if you disagree. Find more about the youtube privacy statement under the following link: https://policies.google.com/privacyFraunhofer IZI employees introduce test viruses into an aircraft cabin. This is followed by extensive virological tests in the laboratory to determine the effectiveness of the measures.
The Fraunhofer Institute for Cell Therapy and Immunology is helping Airbus to develop suitable measures to rid aircraft cabins of viral pathogens (such as SARS-CoV-2).
Electronbeam-based inactivation of viruses and bacteria – the way to the market

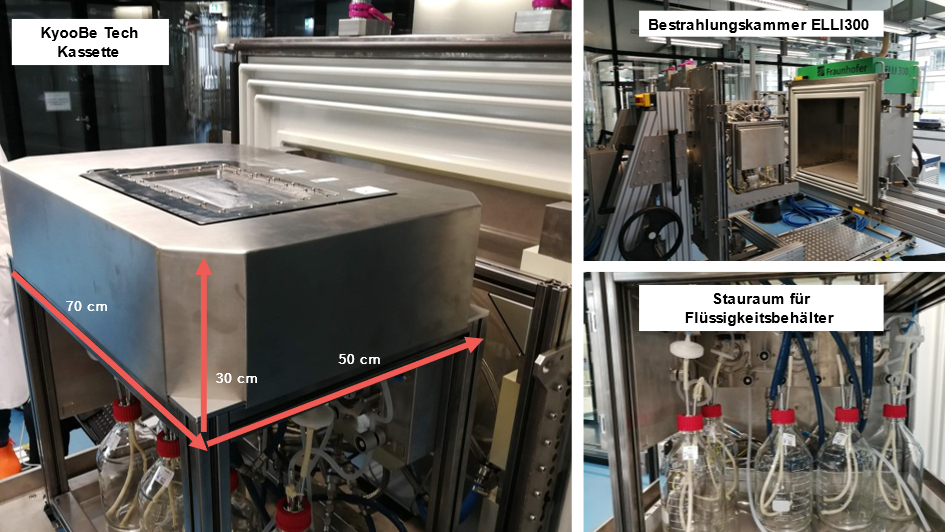
For decades, the production of dead vaccines has been based on killing pathogens by chemicals. Although this method is used for a number of vaccines, it harbors some problems. The chemicals used, such as formaldehyde, are harmful to the environment and health and must be removed before a vaccine can be made from it. In addition, the inactivation process often takes several days to weeks. Since 2014, therefore, together with the Fraunhofer Institutes IPA and FEP, an approach has been pursued that makes the use of harmful chemicals unnecessary and inactivates the pathogens within milliseconds. For this solution, the team received the Fraunhofer Prize ”Technology for People and their Environment” in 2021. Already in 2019, the technology was out-licensed to the filling line manufacturer Bausch & Ströbel, from which the company KyooBe Tech GmbH emerged as a spin-off, which will take over the further development until marketability at the end of 2023.
In 2021, a joint consortium tested concepts and performed test runs that are optimized for manufacturing processes in the pharmaceutical industry. For example, several sensors for different process parameters, e.g. for measuring the temperature, have been integrated. The current cassette prototype is made entirely of pharma-compliant stainless steel. The heart of the cassette is a stainless steel roller over which a thin liquid film is conveyed and irradiated with low-energy electrons via an irradiation window. A scraper system then removes the irradiated liquid from the stainless steel roller and directs it into a separate product container. With the optimized cassette system, between 10 and 20 L/h throughput can currently be produced depending on the type of liquid. This represents an increase of a factor of 10 compared to previous systems. The cassette is also mounted on a carrier module with enough space for various containers and technical equipment.
From 2024, devices should then be commercially available as well as much more compact than the current structure. A device the size of a standard laboratory refrigerator is conceivable. The focus is on the production of vaccines, but an extension to other areas of application such as reducing potential contamination in biological manufacturing processes or producing cell therapeutics is possible.
Fraunhofer Prize for “Human- and Environment-Centered Technology” 2021
For the development of this more efficient, faster and environmentally friendly vaccine production process, the research team is awarded the Fraunhofer Prize "Technology for People and their Environment" 2021.
Press release on the award ceremony / May 5, 2021
This explanatory video shows how the inactivation of viruses and bacteria by low-energy electron radiation works.
Development of an antiviral drug candidate with broad-spectrum efficacy for the treatment of West Nile and Zika flavivirus infections
The FLAVICURE project aims to develop the first broad-spectrum antiviral for treatment of infections caused by the flaviviruses West Nile virus and Zika virus. These flaviviruses are transmitted to mammals and humans by mosquitoes and cause serious diseases. A spread as a result of global warming is becoming apparent, since both the distribution of heat-loving vectors such as the Asian tiger mosquito and the development of the pathogens in the vector are temperature-dependent. Furthermore, West Nile virus is also transmitted by mosquitoes native to central and northern Europe. So far, there are no treatment options and the only preventive strategy is protection against mosquito bites.
The FLAVICURE project is led by Protinhi Therapeutics from Nijmegen, Netherlands. Besides Chimera Biotec GmbH, the Fraunhofer Institute for Cell Therapy and Immunology IZI is involved as a project partner. The Vaccine Technologies and the Preclinical Validation units carry out, among other things, the preclinical efficacy studies of the anitviral lead substances. Based on efficacy and safety, the most promising candidate is then selected and prepared for clinical testing in GLP development studies.
The project is funded by the Eurostars programme, an open-topic funding programme for small and medium-sized companies that carry out joint research and development projects with partners in other member states within the framework of the European research initiative EUREKA (www.eurostars-eureka.eu).
Production of antigens for the development of a serological test for Zika viruses and dengue serotypes
Global warming and increasing globalization and are constantly adding new dimensions to the problems surrounding insect-borne viral infections. The flavivirus genus comprises a number of viruses which are predominantly transmitted by arthropods (ticks and mosquitoes) via birds and mammals. Many of these viruses are harmful to animals and humans, causing diseases such as various forms of encephalitis (including TBE), yellow fever, dengue fever and West Nile fever. The fact that, on a molecular level, the viruses and their subtypes are extremely similar in part makes a differential diagnosis difficult, despite this being an essential prerequisite for targeted therapy. As part of the project, a system is to be developed that will enable serological differentiation between infections caused by the closely related dengue and Zika viruses. The project builds upon Multiplex-based methods for diagnosing vector-borne viral infections. Solutions are to be integrated into the test that will enable the infecting serotype to be accurately identified in the case of dengue viruses. To this end, recombinant antigens will be developed at Fraunhofer IZI which will subsequently be integrated into existing diagnostic procedures, significantly improving specificity.
This project is co-financed by tax revenues on the basis of the budget approved by members of the Saxon state parliament.








