Biological Material Analytics

We test immunomodulatory properties of materials and agents on macrophages.
The Biological Material Analysis Unit develops preclinical, cell-based test models for the biological evaluation of medical products.
The development of biocompatible and immunocompatible medical products, such as medical devices and drugs, requires consideration of the interaction between materials or active substances and biology (biomolecules, cells, and tissues) to be able to use them safely on and in humans.
The Biological Material Analysis Unit therefore pursues an interdisciplinary approach. At the interface of medical device or drug development and immunobiology, materials, substances and active agents are evaluated, as well as in cooperation optimized, or newly developed for industrial partners. Standardized diagnostic screening test procedures are also developed for this purpose.
The focus is on cell-based test scenarios, test systems and analytical procedures for in vitro use. The developed processes form the basic framework for the questions to be investigated. In the implementation, the focus is primarily on physiological processes, i.e., processes in the patient. With such in vitro tests in the preclinical phase, the most suitable materials / active substances for the respective application can be identified and evaluated quickly and reliably, thus accelerating the development of novel medical products. The test scenarios are based on suitable acceptance criteria for method development and validation.
The Fraunhofer IKTS group’s place of work is at the Fraunhofer Institute for Cell Therapy and Immunology IZI in Leipzig. There are close cooperations with groups at the Fraunhofer IZI with the aim to advance medical products and cell therapeutics for industrial applications.
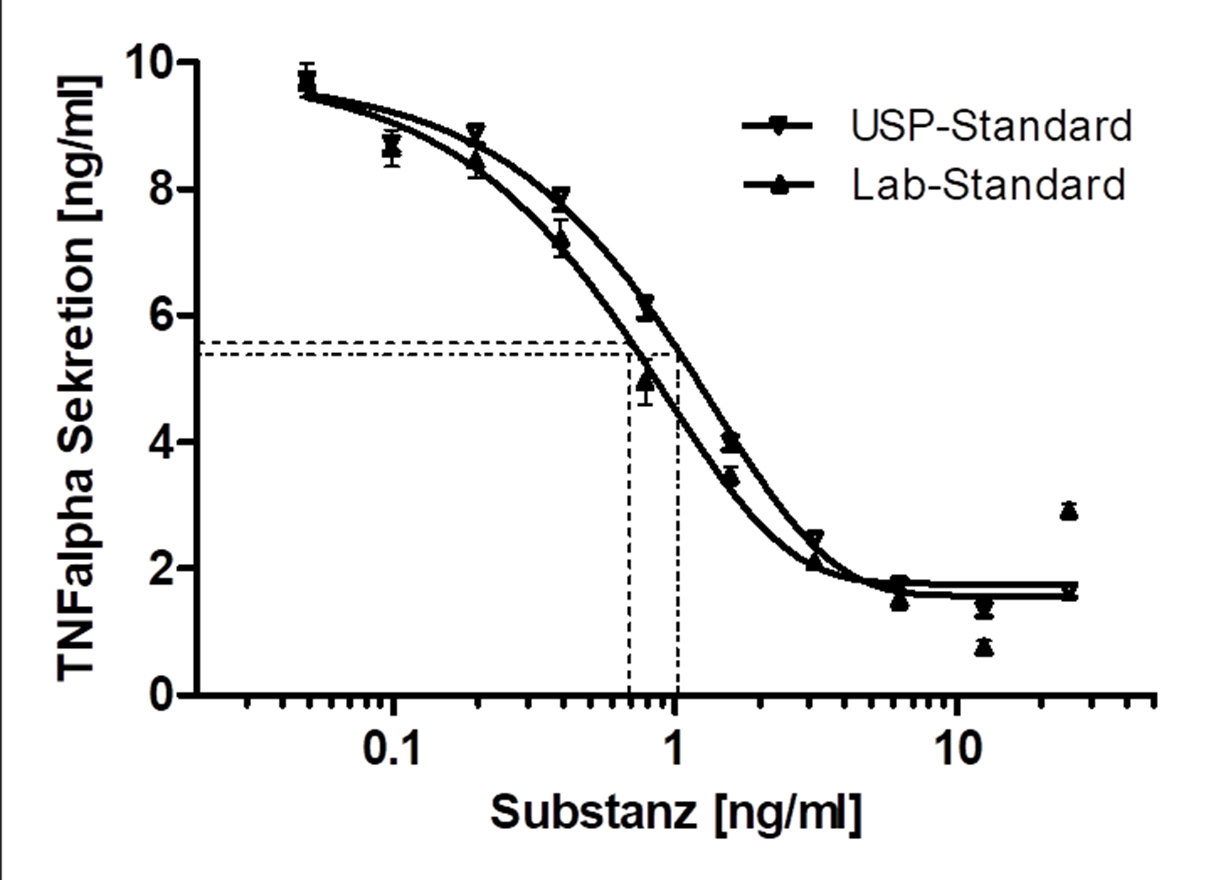
Immune cell-based Potency / Mode of Action assays for drug discovery
In the preclinical testing of drugs, bioassays (Potency Assays) are indispensable to investigate the desired mechanism of action or to validate it in vitro.
Various scenarios were developed in the working group for this purpose:
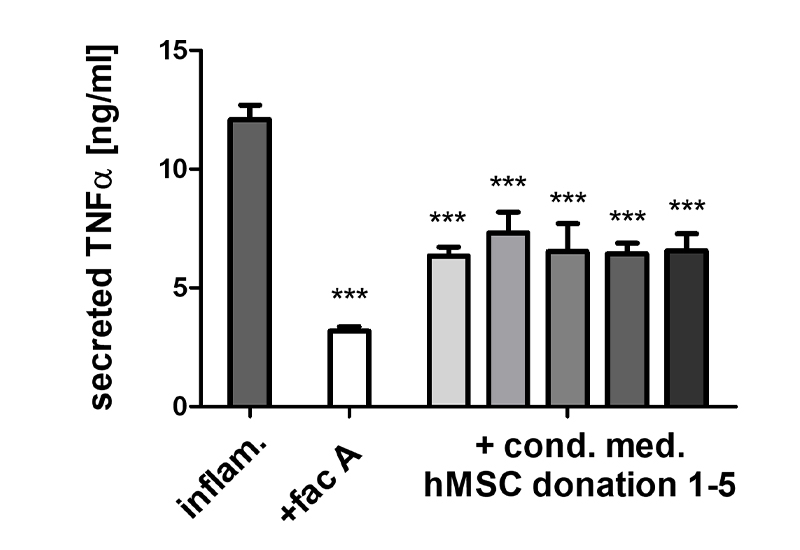
- Because of their key role in inflammatory processes, macrophage-based standardized inflammation models have been established to study different inflammatory or immunomodulatory mechanisms.
- Various test sequences to investigate the interaction with macrophages and/or mesenchymal stem cells (e.g., in new bone formation) are available for Bioassays, Mode of Action, functional or safety analyses.
Topic-adressing projects:
- EU-HORIZON cooperation project with the topic “Next Generation BiOactiVe NAnocoatings“ (Project acronym: NOVA)
- German Korean AiF / ZIM cooperation project to establish a marker-free and specific analysis method for the standardized evaluation of non-transparent biomaterials in macrophage activation (cell holography / laser-assisted cell dissection) – Cooperation project with HICS Company and Pixel Inc. (South Korea), LLS ROWIAK LaserLabSolutions GmbH (Germany) (Project acronym: ImplantHoloCut)
- Dissertations within the Fraunhofer Attract project on the topics "Development of a combined in vitro test procedure for the evaluation of osteo-immunological properties of medical products", "Investigation of the influence of experimental conditions on the differentiation of THP-1 cells into polarized macrophages".
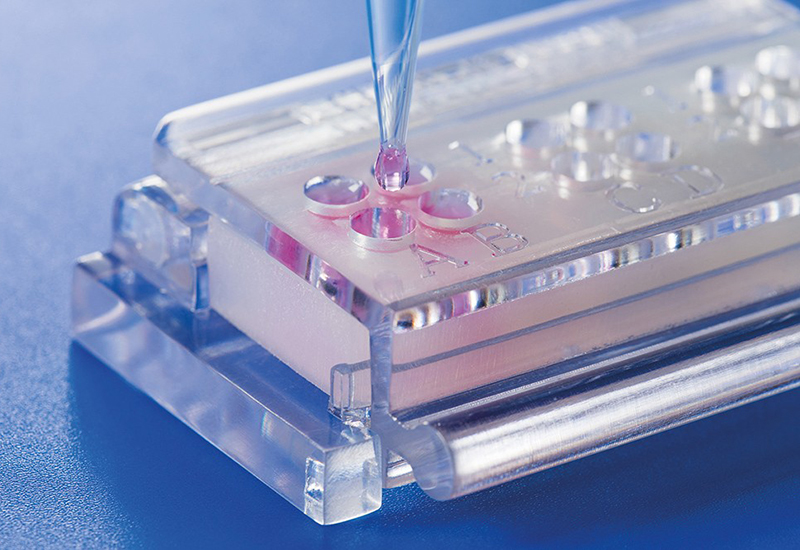
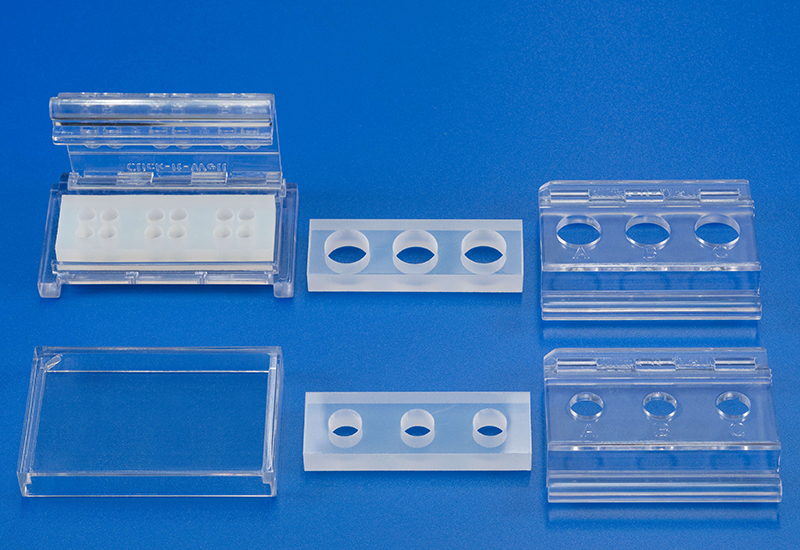
Novel testing system (ClicKit-Well) for biological material testing
Conventionally, materials are examined in vitro in cell culture plates, which makes quantitative comparisons between materials (test specimens), e.g., with different geometries, difficult.
The patented in vitro test system ClicKit-Well was developed for the reliable analysis of surfaces (DE 102018221415 B3). With its surface standardized areas (e.g., 96-well format) on material test specimens, it allows quantitative comparisons between materials.
Topic-adressing projects:
- BMWi - WIPANO project (knowledge and technology transfer through patents and standards) with the aim of developing a quantitative direct cytotoxicity test (as a standard test method) for medical devices – cooperation project with Eurofins BioPharma Product Testing Munich GmbH and DIN
- EU-HORIZON cooperation project with the topic “Next Generation BiOactiVe NAnocoatings“ (Project acronym: NOVA)
- VDE / VDI BMBF VIP+ cooperation project with the topic “Universally usable in vitro testing system for standardized, resource-saving implementation of biointerface studies” (Project acronym: UniBioface)
- Fraunhofer cooperation: IAP, IFAM, ICT within Simpromat (BMBF-funded; funding code: FKZ 01IO1902): Further development of the in-house developed in vitro test system with focus on the serial production of individual components and the application in the field of anti-viral testing.

Privacy warning
With the click on the play button an external video from www.youtube.com is loaded and started. Your data is possible transferred and stored to third party. Do not start the video if you disagree. Find more about the youtube privacy statement under the following link: https://policies.google.com/privacyBiological material analysis for medical products
Establishment of macrophage-based bioassays (Potency assays), screening and Mode of Action assays for service and transfer
- Testing of the immunomodulatory potential of agents, materials, or substances
- Testing of the cytotoxicity of macrophages in the form of active substances, material extracts or in direct cell-material contact
- Immunotoxicological tests (analogous to ISO 10993-10, -20 and -22)
- Cell line based and / or use of primary cells (human)
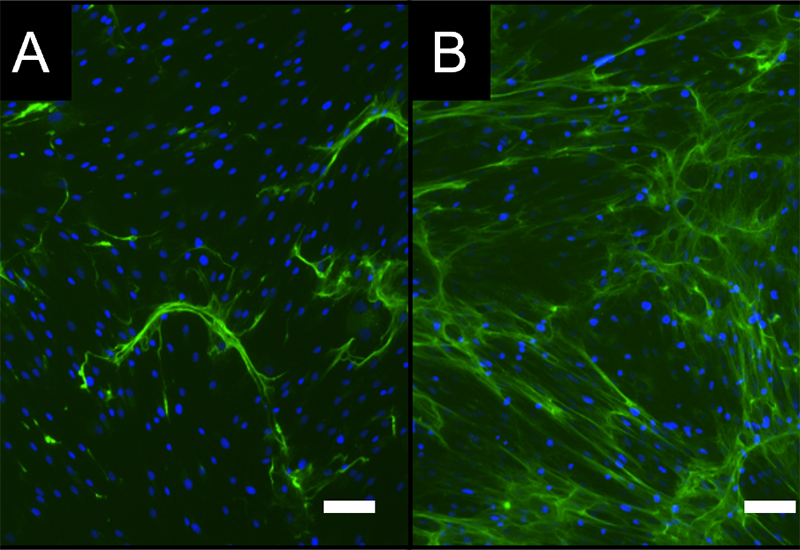
Investigation of the influence of materials and agents on bone formation
- Development / establishment of standardized in vitro test scenarios ("New Bone" assay)
- Combined analysis on osteo-immunological properties of test materials / active substances in bone formation in vitro
- Differentiation assays cell line based or with bone marrow primary cells (human)
Biological assessment of materials (in the context of research and development) analogous to DIN EN ISO 10993
- In vitro cytotoxicity tests analogous to ISO 10993 for the assessment of biocompatibility (extract tests, cytotoxicity / adhesion test in direct cell contact)
Method development
- Development of suitable methods and analytical procedures for the biological evaluation of biomaterials / medical products as well as active substances / drugs for industrially transferable screening assays
- Development of novel immuno- and cell biological assays, e.g., for direct application on material surfaces
- Feasibility and validation studies
- Establishment and validation of marker-free / non-destructive analysis methods
- Development of suitable (depending on the problem) test procedures, incl. controls, and preparation of corresponding protocols (SOPs)
Analytics
- Cell secretome (e.g., ELISA, Luminex)
- Surface markers (e.g., immunofluorescence microscopy, FACS)
- Gene expression (qPCR)
- Enzyme activity determination
- Biochemical assays (e.g., protein, calcium determination)
- MSC characterization according to ISCT criteria (incl. immune potential)
- Non-destructive imaging techniques (including digital holographic microscopy (under development))
Further competences of the Biological Material Analysis Unit:
- Regulatory matters for medical devices (Medical device regulation, EU 745/2017)
- Active participation in ISO 10993 (member of DIN committee NA 027-07-12 AA / expert in ISO TC194, contact person: Dr. Susanne Kurz)
- LEAN principles/process optimization
- New Work